Oncology products monopolise the list of best-selling drugs. With a huge range of indications that is only growing, therapies become more specific to tumour or cell pathologies and are suitable for an increasing number of patients. Several oncology products now have “blockbuster” status where sales exceed $1 billion annually.
This is relevant for clinical trials as more regulators require evidence of efficacy in comparison to the standard of care, which is likely to be one of the blockbuster products. A significant proportion of the late-stage clinical trial pipeline is in oncology indications as immunotherapies and next-generation biotherapeutics are gearing up for approval. This means that sponsors are having to fork out for these blockbuster products in order to run their trials more often.
Keytruda (Pembrolizumab) and Opdivo (Nivolumab)
Merck’s Keytruda ($7.2B) and BMS’s Opdivo ($1.8B) hold the number 2 and number 11 spots, respectively, in annual sales of the top oncology products. They are both checkpoint inhibitors targeted towards programmed cell death protein 1 (PD-1 inhibitors), a protein that is responsible for downregulating the immune system and preventing it from killing cancer cells. As both products target the same pathway, they naturally compete in many indications (Table 1).
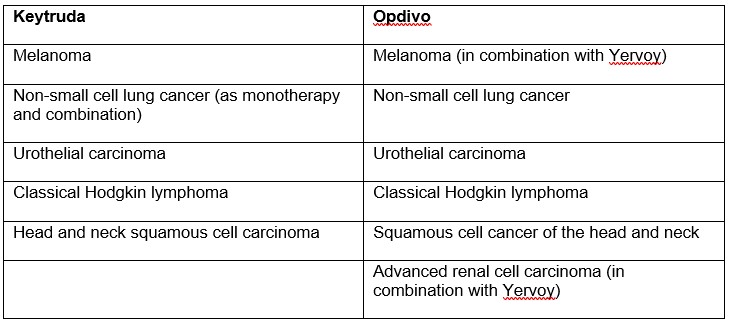
This means for many therapies, it is the physician’s choice whether to use Opdivo (often in combination with Yervoy) or Keytruda. Keytruda has been more successful, in part due to its higher efficacy among all groups and reduced toxicity. Both of these products are incredibly popular in clinical trials; a search on clinicaltrials.gov shows that there are 783 studies recruiting using Keytruda and 707 for Opdivo.
Herceptin (Trastuzumab) and Mabthera (Rituximab)
Roche’s Herceptin and Mabthera come in at number 3 and 5, respectively, in the list of top-selling oncology products, having sales of $7B and $6.8B each. They are highly effective therapies for HER-2 positive breast cancer (Herceptin) and non-Hodgkin’s lymphoma (Mabthera), two of the most common and widely treated cancers.
However, the financial success of these products is under threat. Biosimilars are increasingly becoming the standard of care and both have several licensed biosimilar drugs (e.g. Celltrion’s Herceptin biosimilar, Truxima). As more trials show equivalency in outcomes between the branded and biosimilar product, more purchasers will choose the cheaper option.
On clinicaltrials.gov, there are 246 recruiting studies with trastuzumab and 415 with rituximab; a growing proportion of them will choose the biosimilar. No product is safe from the encroachment of biosimilars; Roche’s Avastin, number 4 on the list of the top-selling oncology products with $6.8B in sales, is also under threat as Amgen’s Mvasi has been approved by the US Food and Drug Administration (FDA).
 As these blockbuster products continue to increase their market share and there are more trials globally that are utilising these products, sourcing them has become a hugely important part of a successful clinical trial. All the products mentioned come at a significant cost, running into the thousands of dollars per dose. It makes sense then to partner with an expert in sourcing to ensure the most efficient, risk-free, and cost-effective solution possible.
As these blockbuster products continue to increase their market share and there are more trials globally that are utilising these products, sourcing them has become a hugely important part of a successful clinical trial. All the products mentioned come at a significant cost, running into the thousands of dollars per dose. It makes sense then to partner with an expert in sourcing to ensure the most efficient, risk-free, and cost-effective solution possible.
Clinical Services International is a trusted partner of leading pharmaceutical and biotechnology companies, contract research organisations, and contract manufacturing organisations. We are experts in sourcing licensed and unlicensed medicines for use in trials, ranging from eye drops to the most sought-after blockbuster therapies.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.









Join or login to leave a comment
JOIN LOGIN