The UK’s National Health Service (NHS) has announced plans to offer Pfizer‘s Voxelotor (Oxbryta), a new treatment option for sickle cell disease patients.
The latest development comes after the National Institute for Health and Care Excellence (NICE) granted approval for the treatment in patients aged 12 years and above.
The decision will benefit up to 4,000 patients in England with the genetic blood disorder.
Voxelotor, which will be available in tablet form for daily intake, can reduce the frequency of blood transfusions and hospital visits.
It offers a new treatment option for those who have had severe reactions to treatment or prefer not to undergo blood transfusions.
The NHS will immediately fund Voxelotor through the Innovative Medicines Fund, ensuring rapid access for patients.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataClinical trials have demonstrated that Voxelotor could improve anaemia and reduce the necessity for repeated blood transfusions.
In trials, 51% of participants treated with Voxelotor experienced an increase in haemoglobin levels, reducing their symptoms and enhancing their overall quality of life.
74% of patients taking the drug reported a significant improvement in their well-being.
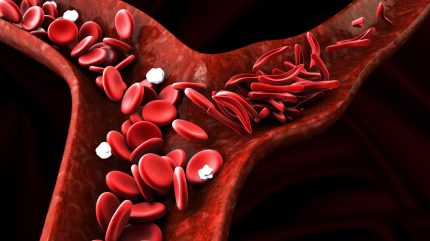
Sickle cell disease is caused by a genetic mutation that results in red blood cells becoming sickle-shaped, leading to haemolytic anaemia, in which red blood cells are destroyed faster than they can be replaced.
NHS chief executive Amanda Pritchard stated: “The NHS has worked hard to make this life-changing treatment available at a fair price for the taxpayer, as part of our wider drive to improve the quality and experience of care for sickle cell patients, and tackle the stigma and inequalities they have told me they face.
“By improving the quality of life and reducing the need for hospital care, this new treatment option also has the potential to free up doctors, nurses and other clinicians to better support other patients, so we are acting fast to get it to the frontline immediately through our Innovative Medicines Fund.”



