
The healthcare and pharmaceutical industries are increasingly adopting advanced technologies to streamline clinical trial management processes.
Current industry challenge with the CTMS:
General Challenges:
- Non-intuitive user interfaces: Clunky interfaces can discourage employees from using the CTMS, leading to incomplete data, inefficient workflows, and reduced overall system value.
- Lack of flexibility: Rigid systems struggle to adapt to changing regulations, trial protocols, and industry best practices. This can lead to compliance issues, wasted resources, and delays.
- High maintenance costs: Frequent updates, patches, and vendor support can significantly inflate the total cost of ownership for legacy systems.
- Scalability limitations: Older systems may not be able to handle the increased data volume and user base demands of large or complex trials, leading to performance issues and system crashes.
- Interoperability and integration challenges: Difficulty connecting with other clinical research software creates data silos, hinders communication, and slows down information sharing across the study ecosystem.
Data Management and Analytics Challenges:
- Outdated reporting capabilities: Limited reporting features make it difficult to analyze trial data effectively, hindering decision-making and slowing down study progress.
- Data silos and integration issues: Lack of seamless integration with other systems leads to data fragmentation, hindering comprehensive data analysis and insights.
- Limited data security: Outdated security protocols may not meet current data privacy regulations, increasing the risk of breaches and non-compliance.
Operational Inefficiency Challenges:
- Manual workflows and paper-based processes: Lack of automation capabilities leads to manual data entry, document management, and communication tasks, increasing errors, delays, and complexity.
- Limited visibility and oversight: Lack of real-time monitoring and reporting functionalities can hinder proactive issue identification and jeopardize patient safety and study timelines.
- Talent acquisition and retention: Outdated interfaces and limited functionalities can make legacy systems unattractive to tech-savvy professionals, hindering team recruitment and retention.
Where does Salesforce comes in this picture:
Salesforce, a leader in the CRM world, surprisingly emerges as a formidable solution for clinical trials.
- Its robust data management model, intrinsic to CRM ⇒ Aligns perfectly with the complexities of trial data.
- The communication tools, pivotal for customer relations ⇒ Translate into enhanced collaboration among trial stakeholders, including researchers, participants, and regulators.
- Advanced analytics and custom workflows, which drive business insights and automate processes ⇒ Ideal for insightful trial monitoring and efficient clinical operations management.
- Salesforce’s scalability and mobile accessibility ⇒ Further adapt it to trials of varying scopes and locations.
In essence, Salesforce’s CRM features, with strategic pivoting, can greatly enhance clinical trial efficiency, compliance, and participant engagement, making it an innovative choice beyond traditional CRM applications.
Here are the top 10 reasons why a Salesforce-based CTMS solution stands out:
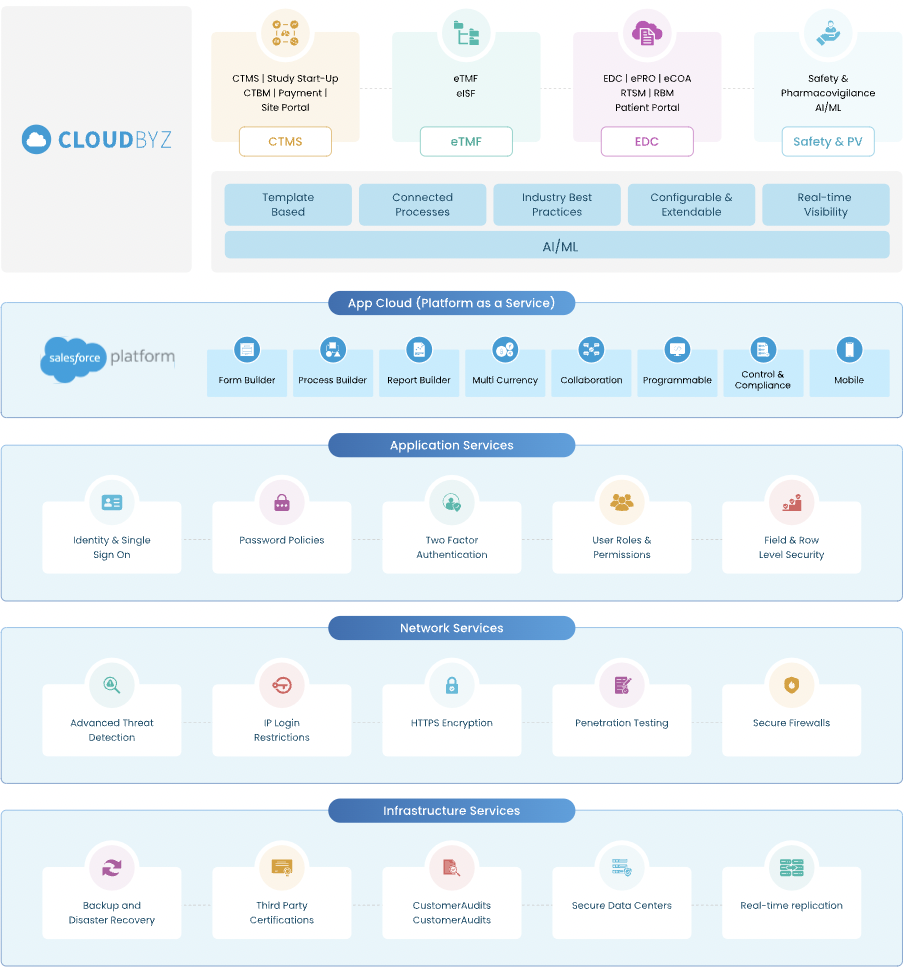
1. Integrated Cloud Platform: Salesforce offers a multi-tenant SaaS platform, ensuring high availability and easy scalability for various customers. This is particularly beneficial for CTMS, which requires reliable, on-demand access to manage complex clinical trials.
2. Enhanced Data Security: With compliance requirements like HIPAA,GDPR, SOC 2,ect, Salesforce provides robust data and information security, critical for handling sensitive clinical trial data.
3. Automatic Disaster Recovery: Salesforce’s automatic setup for disaster recovery and backups ensures the safety and availability of critical trial data.
4. Seamless Scalability: The platform’s scalability is a boon for CTMS, allowing systems to adapt to the varying scope and size of clinical trials.
5. High Availability: Salesforce guarantees a high-availability environment, ensuring CTMS is accessible whenever needed, a must-have for continuous trial monitoring.
6. Collaborative Tools for Enhanced Communication: Tools like Salesforce Chatter promote better communication and collaboration among trial teams, an essential aspect of clinical trial management.
7. Advanced Analytics with Einstein Analytics: Salesforce’s analytics tools provide powerful insights and predictive analytics, enabling more informed decision-making in clinical trials.
8. Easy Integration with Other Systems: Seamless integration with ERPs, CRMs, and other analytics tools makes Salesforce-based CTMS a hub for centralized data management and visibility.
9. User-Friendly Interface for Higher Adoption: Salesforce is known for its intuitive interface, promoting easier adoption and reducing the learning curve for CTMS users.
10. Lower Total Cost of Ownership and Faster Time-to-Value: With lower infrastructure and administrative costs compared to legacy systems, Salesforce offers a more cost-effective and rapidly deployable solution for CTMS.
Transforming Clinical Trial Management
Adopting a Salesforce-based CTMS transforms the way organizations handle clinical trials. It enhances operational efficiency, improves data quality and security, and offers a more user-friendly and scalable solution compared to traditional systems.
Salesforce’s platform is designed to automate and streamline workflows, facilitating better management of clinical trials. Its flexibility and configuration capabilities allow organizations to tailor the CTMS to their specific needs.
In summary, a Salesforce-based CTMS solution offers a comprehensive, secure, and efficient platform for managing clinical trials, ensuring better outcomes, higher compliance, and improved operational efficiency.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com

