FDA approves bispecific drug from Pfizer for multiple myeloma
Bio Pharma Dive
AUGUST 14, 2023
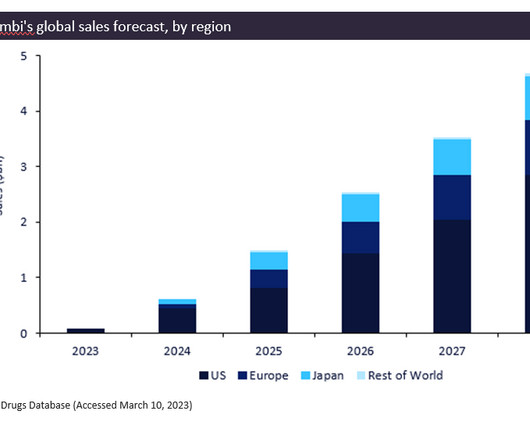
Elrexfio is the third bispecific antibody cleared to treat the blood cancer, and will compete with other therapies that target the “BCMA” protein on myeloma cells.































Let's personalize your content