This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Engineered bacteria find tumors, then alert immune cells
Combining discoveries in cancer immunology with sophisticated genetic engineering, Columbia University researchers have created a sort of "bacterial suicide squad" that targets tumors, attracting the host's own immune cells to the cancer to destroy it. The new work, published today in Science Advances, marks a major step forward in efforts to enlist non-pathogenic bacteria to combat cancer.
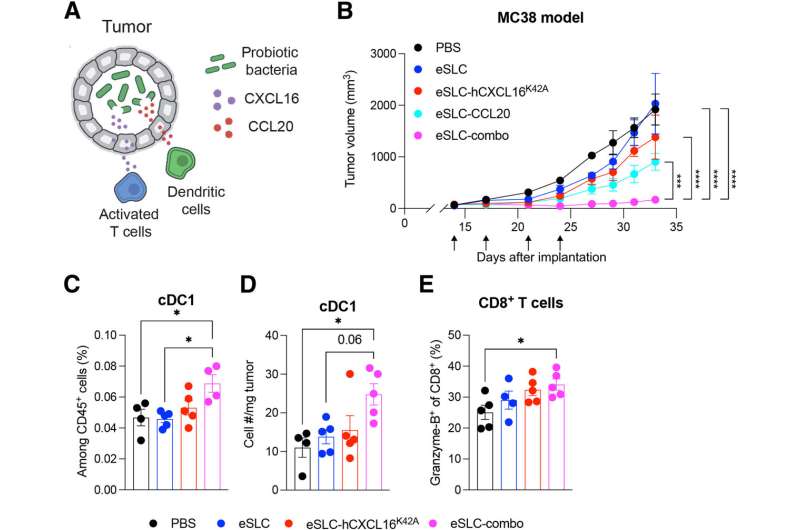
Scientists have known for years that some species of bacteria can thrive inside tumors. "It's been speculated that this is due to the low pH, necrotic and immune-excluded environment … that's unique to the core of a tumor and supports bacterial growth while preventing clearance of bacteria by immune cells," says Nicholas Arpaia, Ph.D., assistant professor of microbiology and immunology at Columbia's Vagelos College of Physicians and Surgeons and senior author on the new paper. In an ongoing collaboration with Tal Danino, Ph.D., associate professor of biomedical engineering at Columbia Engineering, Dr. Arpaia has been building an anti-tumor strategy around that phenomenon.
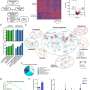
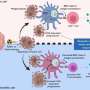
At the core of the approach is a probiotic strain of the bacterium E. coli, engineered with a synchronized lysis circuit. Once the bacterial cells reach a quorum inside a tumor, the circuit triggers, causing most of the bacteria to lyse, or break apart, releasing their contents. Previously, the investigators have added genes to the microbes encoding proteins that block tumor cell growth, or that flag the tumor for digestion by immune cells.
"My graduate student, Thomas [Savage], had the idea of potentially utilizing this platform to deliver chemokines," says Dr. Arpaia, who is also a member of the Herbert Irving Comprehensive Cancer Center (HICCC) at NewYork-Presbyterian/Columbia University Irving Medical Center.
Attracting killer T cells
Immunologists have found that different chemokines, immune system signaling proteins, attract different types of immune cells and stimulate them to respond in specific ways. In the new work, the team included a mutated version of a human chemokine gene that attracts "killer" T cells. "Although T cell responses that are specific to tumor-derived antigens are primed, sometimes what will happen is that despite there being primed anti-tumor T cells, they fail to be recruited into the tumor environment," says Dr. Arpaia.
To further augment therapeutic efficacy, the researchers added a second bacterial strain expressing another chemokine, this time to attract dendritic cells. "By coupling this with chemokines that drive the infiltration and activation of dendritic cells, a critical innate immune cell type, detection of tumor antigens is increased," says Dr. Arpaia. Activated dendritic cells eat the tumor cells, then present their antigens to the T cells, which can then recognize the tumor cells better and respond to them more reliably.
The new work involved collaborators from the Department of Pathology and Cell Biology, the HICCC and the Data Science Institute at Columbia, and also built on a long series of previous findings by others. "Through decades of research that's allowed us to understand how an immune response develops, [we're] developing therapeutics that specifically target each one of those discrete steps," says Dr. Arpaia.
In mouse models of cancer, the engineered bacteria induce robust immune responses against tumors that have been injected directly with the bacteria, as well as more distant tumors that weren't injected. Delivering the bacteria intravenously also works. "What we see is that the bacteria will only colonize the tumor environment, and they only reach a sufficient level of quorum to induce lysis within the tumor, so we can't detect bacteria in other healthy organs," says Dr. Arpaia.
The scientists continue to tinker with the system to optimize it, while also laying the groundwork to take it into clinical trials. Dr. Arpaia and some of his collaborators have applied for a patent on the approach, and are part of a company, GenCirq, Inc., to develop the therapy further.
More information: Thomas M. Savage et al, Chemokines expressed by engineered bacteria recruit and orchestrate antitumor immunity, Science Advances (2023). DOI: 10.1126/sciadv.adc9436