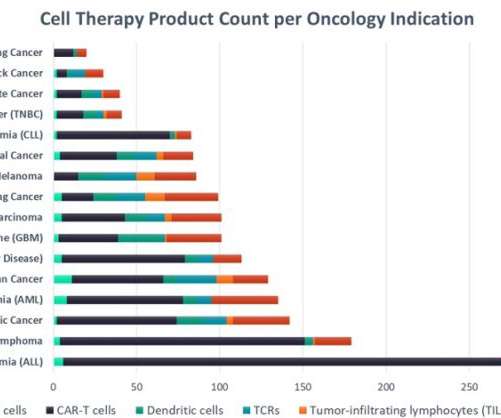
Acute lymphocytic leukaemia leads in cell therapy clinical development
Pharmaceutical Technology
JULY 12, 2022
Despite the market size for B-cell non-Hodgkin’s lymphoma (NHL) being at least five times that of ALL, NHL has a smaller pipeline of cell therapies. The total market for cell therapies in oncology is projected to exceed $37bn worldwide by 2028.







































Let's personalize your content