Signant clinical supply chain software offers end-to-end visibility
Outsourcing Pharma
SEPTEMBER 22, 2021
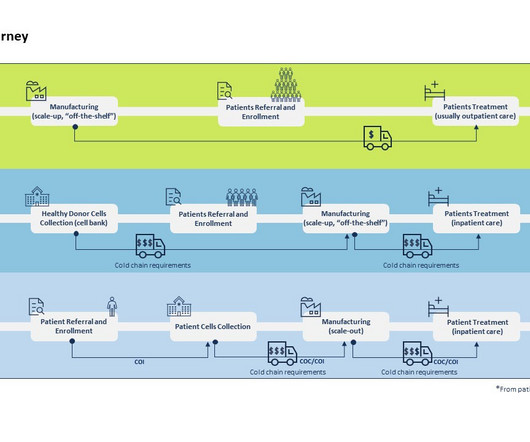
SmartSignals Supplies is designed to help trial teams get a handle on clinical trial supply chain management to avoid delays, waste, and cost overruns.


















Let's personalize your content