US FDA approves Regeneron-Sanofi’s Kevzara to treat polymyalgia rheumatica
Pharmaceutical Technology
MARCH 1, 2023
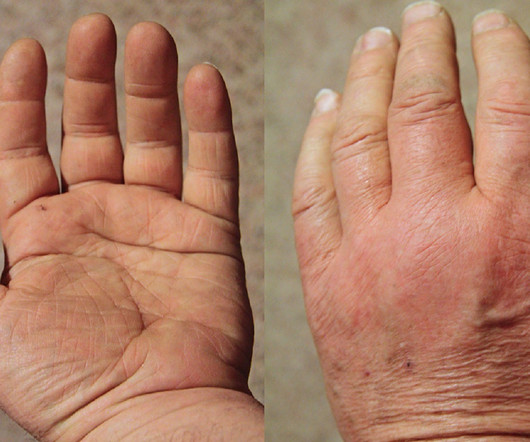
“With the approval of Kevzara for polymyalgia rheumatica, patients now have an FDA-approved treatment to help offer relief from the disabling symptoms of this disease and long-term dependence on steroids.”
































Let's personalize your content