“Decentralized clinical trials (DCTs) were critical in enabling clinical research activities during the pandemic,” notes Adam Samson, MS, PMP, CCRA, CCRC, CCDM, Head of Clinical Delivery Operations, Real-World Evidence Clinical Trials, at Walgreens. “Yet, this approach—where some or all trial-related activities occur at locations other than traditional clinical trial sites—is not the sole component for modernizing clinical research.”
Post pandemic, there are signs of “DCT fatigue,” with much of the industry returning to in-person visits at brick-and-mortar sites only, and with the shift from paper records to electronic systems at sites slowing a bit, notes Samson. “Continued progress in modernizing clinical trials is essential,” he adds. “This should include evaluating appropriate use of technology and the potential of non-traditional sites, such as retail pharmacies, as well as offering at-home services to study participants.”
Samson asserts that clinical trial stakeholders should aspire to elevate the patient experience of study participation. “There may be issues involving lack of study awareness and access among underserved communities, or a need to travel long distances to study sites,” he says. “We should aim for a high-quality, end-to-end patient experience, based on learnings from sectors with advanced consumer offerings such as banking, travel, and entertainment. Implementing new solutions requires care to ensure positive impacts on the patient.”
Key steps include fully understanding the patient journey and the workflows required by protocols in place. As Samson explains, these are often clear-cut for site-based trials. They become more complex with decentralized elements, which might include distribution of study drugs directly to patients, use of mobile phlebotomy services, or remote monitoring of safety events. Journey mapping enables risk assessments to be carried out in multiple areas. Gaps and risks can then be identified and addressed.
“Modernizing clinical research should effectively drive down the cost of drug development by accelerating timelines and making better use of resources; for example, by studying multiple drugs within a single protocol,” states Samson. “Decentralized trial elements have potential to support these efforts, but there remains a need for more published literature describing experiences and outcomes. Efficiencies can potentially reduce drug prices once these investigational products reach the market, providing additional benefit to patients.”
Progress is being made towards industry alignment on how to perform patient journey and workflow mapping, such as a recent initiative from the Decentralized Trials and Research Alliance (DTRA), concludes Samson. “Additional efforts should include development of tools to help sites work with vendors in the DCT environment,” he says. “Precompetitive collaborations through such organizations as ACRP and the Clinical Trials Transformation Initiative are also playing key roles in advancing the modernization of clinical trials. Aligning on common principles and goals established by these industry groups would be an excellent step forward to help maintain innovation and resilience.”
Catherine Gregor: DCTs are Following a Hype Cycle
“I agree that we are seeing ‘DCT fatigue’ among trial stakeholders,” says Catherine Gregor, MBA, CCRP, CCRC, Chief Clinical Trial Officer, Florence Healthcare. “DCTs seem to be following the Gartner hype cycle, where unrealistic expectations are seen before successful approaches ultimately gain acceptance.” This cycle follows a maturation curve with several steps—innovation trigger, peak of inflated expectations, trough of disillusionment, slope of enlightenment, and plateau of productivity. DCTs are currently in the trough of disillusionment, due to unrealistic expectations established during the pandemic.
Hype cycle for emerging technologies, 2023 (Gartner)¹
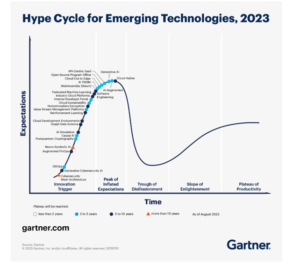
“Today, we’re dealing with frustration due to sites being asked to implement multiple new technologies that add to the burden of entering data into the system,” explains Gregor. “These solutions have often been designed primarily with sponsor or contract research organization (CRO) convenience in mind, and without enough consideration of user needs at the site level. Sponsors are starting to acknowledge the need to make it easier for sites to work with them.”
“Moving forward, we need a clinical trial ecosystem that does not disproportionately burden one set of stakeholders—in this case the sites,” notes Gregor. “The changed workflows needed by new technologies come with a cost. This has been absorbed by sites, despite the fact that almost all benefits accrue to sponsors and CROs. To reflect this, funding of change management should be the responsibility of sponsors and CROs. These organizations should work together to coordinate the demands they make on sites. This may help advance tools such as shared sponsor portals.”
“Over the next five years, vendors also need to collaborate to make their offerings interoperable, paving the way to derive more benefit from digitizing data and workflows,” says Gregor. “This could mirror successes seen in sectors such as banking, where financial data can be transferred across systems and institutions in a process that appears seamless to the consumer. Consolidation of vendor offerings has potential to make decentralized technology elements sustainable, and to avoid the risk that sites will return to familiar, low-tech processes such as paper record-keeping.”
“We need to move beyond the term, ‘DCTs,’ which is polarizing, and talk more broadly about how to modernize clinical trials using technology, processes, people, and physical sites,” concludes Gregor. “All stakeholders will benefit from smart decisions about where to apply their efforts.”
Mo Ali: Sites Bear an Unsustainable Burden
“I agree that there is a sense of fatigue at sites due to the high levels of change management, integration, and support required for DCTs,” says Mo Ali, Treasurer of the ACRP Board of Trustees. “Sponsors have been pushing for fully decentralized trials using multiple innovations within one trial—such as artificial intelligence, remote shipping, and home health nursing—with the goal of improving the efficiency of drug development by faster delivery of trials.”
“This places an unsustainable burden on the sites that must navigate these technologies on behalf of the sponsor,” notes Ali. “Site staff are saying that they cannot implement DCTs due to lack of resources, risking a loss of valuable, long-term relationships between experienced sites and sponsors.”
“There is a shift of mindset among some sponsors, recognizing the need for improvements within their own processes before demanding additional efficiencies from other study stakeholders,” according to Ali. “There are many opportunities to eliminate inefficiencies at the sponsor level, in areas such as protocol development and delivery, and the rollout of documentation, systems, and processes. Greater automation may be possible in these areas.”
Looking Beyond DCTs for the Modernization of Clinical Research
Join Catherine, Mo, and Adam at ACRP 2024 [May 3 – 6; Anaheim, CA], as they discuss common challenges and pitfalls encountered when implementing new research models. View complete schedule.

“We have yet to determine whether the best way to achieve efficiencies is through fully decentralized or hybrid studies,” notes Ali.
In addition to potential for efficiencies, decentralized study elements can support the regulatory mandate for improved diversity in clinical trial populations, improving accessibility for participants without access to bricks-and-mortar sites. “To achieve this promise, however, it will be important to understand what would make these underserved populations want to participate in trials,” Ali points out. “A hybrid format could help ease the burden of patient participation, expanding access to studies. The motivation to enroll may be highest in therapeutic areas such as oncology, but could also be improved in other areas based on a better understanding of standards of care and best practices, including in countries outside the United States. Advances in infrastructure for remote study elements during the pandemic can be leveraged.”
Ali concludes that “hybrid studies gained traction among sponsors in the second half of 2023 as part of a trend that is likely to continue this year and into 2025. It will take time to gather enough evidence to understand what the best hybrid model looks like for each disease area and country, but I’m confident that we are heading in the right direction.”
Reference
Edited by Jill Dawson



