
Clinical trials are essential for the development of new therapies and treatments, and they require the coordination of multiple stakeholders, including study sponsors, investigators, patients, and regulatory agencies. To manage the complex process of a clinical trial, various eClinical vendors supply platforms that help in electronic data capture (EDC), electronic patient-reported outcomes (ePRO), clinical trial management systems (CTMS), electronic clinical outcome assessments (eCOA), clinical trial supply management (CTSM), and randomization and trial supply management (RTSM). However, these eClinical platforms come with unique challenges that impact clinical trial operations.
- EDC Platforms:
EDC platforms allow for the capture of clinical trial data in real-time, which increases efficiency and reduces the likelihood of errors. However, these platforms can present challenges related to system compatibility, data security, and data transferability. If the EDC system is not compatible with other systems used in the clinical trial, data transfer and analysis can be problematic, leading to potential delays in the trial.
- ePRO Platforms:
ePRO platforms allow for the capture of patient-reported outcomes electronically, which can reduce errors and improve data quality. However, challenges arise in terms of patient engagement and compliance. Patients may be reluctant to use unfamiliar technology or may have difficulty navigating the platform. Additionally, patients may not be consistent in reporting outcomes, leading to missing data or data that is difficult to interpret.
- CTMS Platforms:
CTMS platforms allow for the management of clinical trial activities, including monitoring, data management, and study site management. Challenges arise when multiple CTMS platforms are used within a single trial or when CTMS platforms are not integrated with other eClinical systems. This can lead to issues with data synchronization, delays in data analysis, and communication problems between stakeholders.
- eCOA Platforms:
eCOA platforms allow for the capture of clinical outcomes electronically, including clinical assessments, quality of life measures, and other clinical endpoints. Challenges arise with eCOA platforms when the technology is not user-friendly, leading to difficulty in capturing data accurately. Additionally, data may be lost if the platform experiences technical issues.
- CTSM Platforms:
CTSM platforms allow for the management of clinical trial supplies, including inventory management, logistics, and distribution. Challenges arise when supply chain disruptions occur, or when there is a lack of visibility into supply levels or demand. This can lead to delays in the trial, additional costs, and potentially, patient safety concerns.
- RTSM Platforms:
RTSM platforms allow for randomization and trial supply management, which is critical for the success of clinical trials. Challenges arise when RTSM platforms are not integrated with other eClinical systems, leading to data transfer issues, data discrepancies, and communication problems between stakeholders.
While eClinical platforms offer many benefits in managing clinical trials, they come with unique challenges that can impact clinical trial operations. These challenges include system compatibility, data security, data transferability, patient engagement, compliance, user-friendliness, supply chain disruptions, and integration with other eClinical systems. To mitigate these challenges, it is essential to select eClinical vendors that offer high-quality and integrated eClinical solutions and have experience in managing clinical trials.
How to address these challenges
Clinical trials are essential for the development of new therapies and treatments, but they require the coordination and management of multiple stakeholders. eClinical platforms, such as EDC, ePRO, CTMS, eCOA, CTMF, and RTSM, are crucial in managing clinical trial data, processes, and supplies. However, these platforms come with unique challenges that can impact clinical trial operations. In this article, we will discuss how to address these challenges and ensure the success of clinical trials.
- Address System Compatibility
One of the most significant challenges in managing eClinical platforms is system compatibility. Different systems may use different protocols, formats, or data structures, which can lead to issues with data transfer and analysis. To address this challenge, it is essential to ensure that eClinical platforms are compatible with each other and with the data analysis tools used in the trial. This can be achieved by selecting eClinical vendors that offer open systems architecture and have experience in integrating different systems.
- Ensure Data Security
Data security is critical in clinical trials to protect patient privacy and prevent data breaches. eClinical platforms must be designed with robust security measures, including encryption, access controls, and data backup. To address this challenge, it is important to select eClinical vendors that comply with data security standards, such as HIPAA, GDPR, and ISO 27001. Additionally, it is essential to provide training to stakeholders on data security best practices, such as password management, device security, and secure data transfer.
- Ensure Data Transferability
Data transferability is another challenge in managing eClinical platforms. Data must be transferred securely and efficiently between different systems and stakeholders. To address this challenge, it is important to establish data transfer protocols, such as using standard data exchange formats, data dictionaries, and data mapping tools. Additionally, it is essential to ensure that data transfers are auditable, and stakeholders have access to the data they need when they need it.
- Address Patient Engagement
Patient engagement is critical in clinical trials to ensure that patients are compliant with study protocols and that the data captured is accurate and complete. ePRO platforms are crucial in capturing patient-reported outcomes electronically, but patients may be reluctant to use unfamiliar technology or have difficulty navigating the platform. To address this challenge, it is essential to provide patients with training and support on how to use the ePRO platform. Additionally, it is important to design ePRO platforms that are user-friendly, accessible, and have patient-centric features, such as reminders, feedback, and gamification.
- Address Supply Chain Disruptions
Clinical trial supplies are critical in ensuring that the trial runs smoothly and that patients receive the necessary treatment. CTSM platforms are essential in managing the supply chain, but supply chain disruptions can occur, leading to delays and potentially patient safety concerns. To address this challenge, it is important to establish robust supply chain management processes, such as inventory management, demand forecasting, and contingency planning. Additionally, it is essential to work with reliable suppliers and logistics providers and to establish clear communication channels with all stakeholders involved in the supply chain.
- Address Integration Issues
Integration issues are a significant challenge in managing eClinical platforms. Different systems may use different data structures, leading to issues with data synchronization and communication between stakeholders. To address this challenge, it is important to establish integration standards, such as using open systems architecture, standard data exchange formats, and data mapping tools. Additionally, it is essential to work with eClinical vendors that have experience in integrating different systems and can provide technical support and training to stakeholders.
eClinical platforms are critical in managing clinical trials, but they come with unique challenges that can impact clinical trial operations. Addressing these challenges requires a combination of technical solutions, such as open systems architecture, data transfer protocols, and data security measures, and stakeholder
How Cloudbyz Unified Clinical Trial Management Platform Can Solve these challenges
Addressing the challenges of managing eClinical platforms requires a comprehensive approach that includes technical solutions and stakeholder engagement. One solution that can help address these challenges is the use of a unified clinical trial management platform that brings all the capabilities of EDC, ePRO, CTMS, eCOA, ETMF, and RTSM into a single platform.
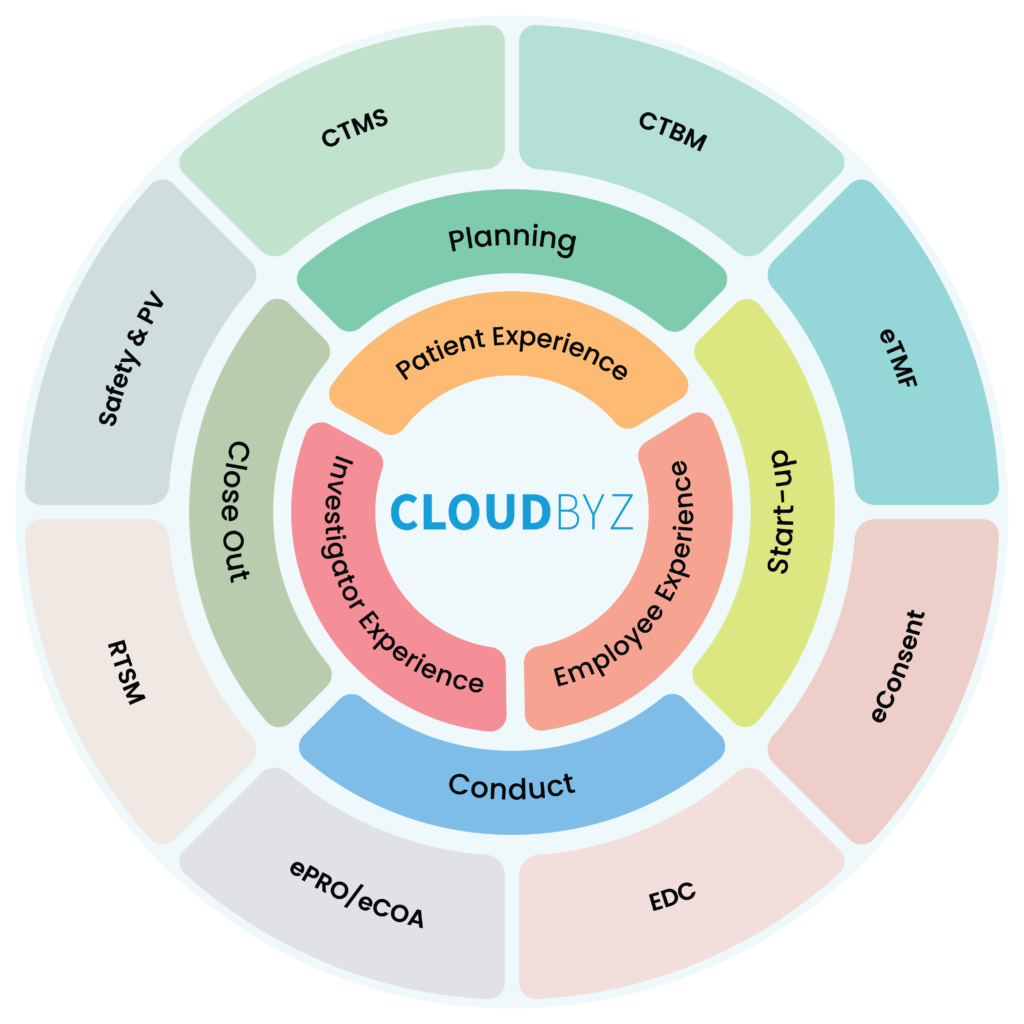
The Cloudbyz Unified Clinical Trial Management (UCTM) platform is an example of such a platform that can help address these challenges.
- System Compatibility
Cloudbyz UCTM platform uses an open systems architecture that allows for easy integration with different eClinical systems used in the trial. The platform is compatible with standard data exchange formats, such as CDISC, and can integrate with data analysis tools, such as SAS, R, and SPSS.
- Data Security
The Cloudbyz UCTM platform is designed with robust security measures, such as encryption, access controls, and data backup. The platform complies with data security standards, such as HIPAA, GDPR, and ISO 27001, and provides training to stakeholders on data security best practices.
- Data Transferability
The Cloudbyz UCTM platform provides data transfer protocols, such as using standard data exchange formats, data dictionaries, and data mapping tools. The platform allows for auditable data transfers and provides stakeholders with access to the data they need when they need it.
- Patient Engagement
The Cloudbyz UCTM platform is designed with patient-centric features, such as reminders, feedback, and gamification, to improve patient engagement and compliance. The platform provides training and support to patients on how to use the ePRO platform and ensures that the platform is user-friendly and accessible.
- Supply Chain Disruptions
The Cloudbyz UCTM platform provides robust supply chain management processes, such as inventory management, demand forecasting, and contingency planning, to address supply chain disruptions. The platform works with reliable suppliers and logistics providers and establishes clear communication channels with all stakeholders involved in the supply chain.
- Integration Issues
The Cloudbyz UCTM platform provides a unified platform that eliminates integration issues between different eClinical systems used in the trial. The platform uses an open systems architecture and standard data exchange formats to ensure data synchronization and communication between stakeholders.
The use of a unified clinical trial management platform, such as the Cloudbyz UCTM platform, can help address the challenges of managing eClinical platforms. The platform provides technical solutions, such as system compatibility, data security, data transferability, patient engagement, supply chain management, and integration, and engages stakeholders to ensure the success of clinical trials.
Conclusion
Clinical trials are crucial for developing new therapies and treatments, but managing the complex process of a clinical trial requires coordination of multiple stakeholders and eClinical platforms, such as EDC, ePRO, CTMS, eCOA, eTMF, and RTSM. These platforms come with unique challenges, such as system compatibility, data security, data transferability, patient engagement, supply chain disruptions, and integration. Addressing these challenges requires a comprehensive approach that includes technical solutions and stakeholder engagement. One solution is the use of a unified clinical trial management platform, such as the Cloudbyz Unified Clinical Trial Management (UCTM) platform, which provides a comprehensive solution to address these challenges. The Cloudbyz UCTM platform uses an open systems architecture, provides robust data security measures and data transfer protocols, incorporates patient-centric features, provides supply chain management processes, and eliminates integration issues between different eClinical systems. Using a unified clinical trial management platform can streamline the clinical trial process and ensure the success of clinical trials.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com


