Being the second most populous country in the world with skilled labor and good infrastructure, India is a favorable destination for clinical trials for a wide variety of diseases. However, it is necessary to develop rules that help streamline regulations and foster clinical trial conduct in India and ensure transparent and ethical practices. With the aim of making India a preferred destination for clinical trials, the Ministry of Health and Family Welfare (MoHFW), Government of India has released a comprehensive set of rules known as the New Drugs and Clinical Trials Rules, 2019 (NDCT) that include 13 chapters and eight schedules are meant to supersede Part XA and Schedule Y of the Drugs and Cosmetics Rules, 1945.
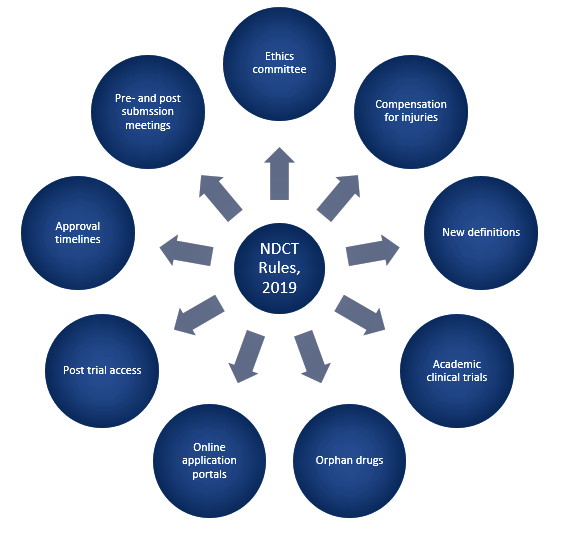
Figure 1 depicts the key changes introduced in the NDCT rules, 2019.
Figure 1. Noteworthy changes in NDCT Rules, 2019
Ethics Committee (EC):
The rules specify a change to the composition, membership, duties, and functioning of the EC. Increasing the percentage of nonaffiliated members in the EC helps decrease bias and facilitates better decision-making. It is necessary for EC members to be trained periodically on Good Clinical Practice (GCP) guidelines such that they are updated on the latest regulations and changes. Other requirements include inclusion of female members for diversity, application for registration and approval of EC with the authority designated by the MoHFW, notification of changes in membership to EC to the Central Licensing Authority (CLA) within 30 days, validity period of registration of 5 years, maintenance of records for 5 years following completion of clinical trial or BA/BE study, and reasons for suspension or cancellation of EC by the CLA. The responsibilities of the EC include protection of the rights, safety, and well-being of trial subjects including vulnerable groups, maintenance of standard operating procedures (SOPs), and review of trial progress through progress reports and protocols. The rules also allow for the simultaneous submission and approval process for clinical trials by the CLA and the EC.
Compensation for injuries:
This part includes information on provisions related to compensation for injuries or death during a clinical trial or BA/BE study and medical management of subjects. It is imperative for the Sponsor to provide free medical management for the appropriate time or until it is proven that the injury is unrelated to the clinical trial. In case of serious adverse events (SAE) or injuries it is necessary for the Sponsor and the investigator to provide a report to the Drugs Controller General of India (DCGI) and EC within 14 days of knowledge of the SAE. The quantum of compensation is calculated based on the formula in the Seventh Schedule of the NDCT rules. In case of death related to the trial or BA/BE study, the Sponsor or representative is required to pay the compensation specified in the order received by the CLA within 30 days.
Definitions:
New and revised definitions are available for terms such as new drug, academic clinical trial, BA/BE study, efficacy/effectiveness, orphan drug, phytopharmaceutical, post-trial access, similar biologic, sponsor, etc. These changes are intended to provide better clarity. Of importance is the definition of new drug which has been revised to include phytopharmaceuticals, novel drug delivery system of any drug, and living modified organism, monoclonal anti-body, stem cell-derived products and gene therapeutic products or xenografts, intended to be used as drugs. All drugs other than NDDS and vaccines, recombinant products, monoclonal antibody, xenografts, etc would be new drugs for four years from the date of permission granted by CLA whereas the mentioned ones would always be new drugs.
Academic clinical trials:
These are clinical trials of a drug already approved for a certain claim and initiated by any investigator and academic or research institution for a new indication, route of administration, dose, or dosage form and where the results are to be sued only for academic or research purposes and not for seeking approval of the CLA or regulatory authority of any country. For these trials, no approval is required by the CLA and only EC approval is necessary, and the trial should be conducted in accordance with the approved protocol and ethical principles specified in the National Ethical Guidelines for Biomedical and Health Research Involving Human Participants. Any conflict between a clinical trial and an academic clinical trial should be notified by the EC to the CLA for examination and final decision making.
Orphan drugs:
To promote research on orphan drugs (drug intended to treat a disease which affects not more than five lakh persons in India), the rules specify waiver for application fees, provision for fast track approval process and an expeditious review process following evidence of clinical safety and efficacy for drugs for rare diseases in which no alternate treatment is available.
Online application portal:
The NDCT rules reference several online forms available via the SUGAM portal to facilitate application and approvals for clinical trials and BA/BE studies. This digital platform enables an easy application and tracking system via real-time updates on submissions. Some noteworthy forms include Form CT-04 (permission to conduct a clinical trial for a new drug or investigational new drug(IND)), Form CT-05 (application for permission for a BA/BE study), Form CT-08 (application for permission for BA/BE study center), application for permission for manufacture of new drugs or investigational new drugs for clinical trials or BA/BE studies (CT-10), application for permission for import of new drugs and INDs for clinical trials, BA/BE studies, examination, test or analysis, and application for permission for import or manufacture of a new drug for sale or distribution (CT-18). Forms for grant of permission for BA/BE study (CT-07), BA/BE study centers (CT-09), manufacture of new drugs or INDs (CT-11), import of drugs for trials and BA/BE study (CT-17), and import for sale or distribution (CT-19 or CT-20).
Post-trial access:
This means making a new drug or IND available to a trial subject after completion of a clinical trial through which the drug has been found beneficial to the trial subject during a clinical trial, for a period considered necessary by the investigator and EC. The Sponsor must provide the drug free of cost upon approval by the EC in cases and for indications for which there is no alternate therapy and informed consent has been obtained. However, it is important to note that the Sponsor is not liable for post-trial use for the drug.
Review and approval timelines:
The time limit for the CLA to respond following receipt of a submission for application for clinical trials has been changed. In case of drugs developed outside India, there is a 90-day limit for the CLA to respond (approval or rejection), and for drugs developed in India this time is decreased to 30 days following which if there is no communication received from the CLA, permission for the clinical trial will be deemed to be granted. This change has helped to foster drug development and clinical trial conduct in India as the timelines are relatively short and predictable.
Pre-submission meetings:
The NDCT rules have included a provision for
pre-submission meetings with the CLA following submission of an application and accompanying fees. These meetings help Sponsors and investigators seek guidance on laws and develop suitable regulatory strategies for product registration and submission and improve transparency and efficiency.
It is expected that the new rules will help protect patient safety and well-being, decrease bias in trials, expedite trials, and foster indigenous drug development as well as attract global clinical trial operations in India. Certain amendments and further clarifications to the rules may be necessary for better understanding and sound application of the rules.
References
- faqnd.pdf (cdsco.gov.in)
- NewDrugs_CTRules_2019.pdf (cdsco.gov.in)
- New drugs and clinical trials rules 2019: Changes in responsibilities of the ethics committee – PMC (nih.gov)
- IndJPhaEdRes_53_4s_451_0.pdf (ijper.org)
- https://www.ijclinicaltrials.com/index.php/ijct/article/download/450/251/1915