UK MHRA publishes guidance on medicines containing valproate
Pharmaceutical Technology
OCTOBER 12, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has published updated guidance on valproate-containing medicines.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Pharmaceutical Technology
OCTOBER 12, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has published updated guidance on valproate-containing medicines.

XTalks
NOVEMBER 21, 2023
This episode features an interview with Attorney Temitope (Tope) Leyimu, a toxic exposure attorney at Motley Rice who is leading a lawsuit over hair relaxing products that contain harmful chemicals. The FDA recently issued a recommendation for the recall of chemical hair relaxers containing harmful chemicals. Temitope (Tope) O.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Scienmag
MAY 4, 2022
Seems like kids are always getting into something, so products marketed toward them often claim to repel liquids. Some items contain potentially harmful per- and polyfluoroalkyl substances (PFAS) to accomplish this feat, but companies aren’t required to disclose these “forever chemicals” on labels.

The Pharma Data
NOVEMBER 30, 2020
30, 2020 – Nearly 15% of talc-based cosmetic products analyzed in a recent study contained asbestos. She noted that EWG’s online database has identified more than 2,000 personal care products that contain talc, including more than 1,000 loose or pressed powders that could pose an inhalation risk. MONDAY, Nov.

Drug Discovery World
AUGUST 4, 2022
It may seem counterintuitive to spend time and money on planning for containment and delivery systems for a drug in the earliest stages of discovery when the chances of that molecule making it to market are still quite low. The increasing complexity of drug products has raised concern among pharmaceutical developers. Never too early.

Roots Analysis
FEBRUARY 28, 2022
Considering patient’s health and safety, the drug products are expected to be free from microbial contamination and should be safe for use throughout the drug’s life cycle. Therefore, several quality checks are performed to ensure product’s sterility and stability. Evolving Landscape of Container Closure Integrity Testing Services.
Bio Pharma Dive
MARCH 20, 2023
Speeding up the drug development process through a less optimized containment and delivery system may have been an option in the past, but both expectations and the competitive environment are changing.

Medical Xpress
MARCH 13, 2023
A new study in the Journal of the Academy of Nutrition and Dietetics has determined that 60% of foods purchased by Americans contain technical food additives including coloring or flavoring agents, preservatives, and sweeteners. This represents a 10% increase since 2001. in 2001 to 4.5 in 2019. in 2001 to 4.5 in 2019.

Medical Xpress
MAY 11, 2023
Tens of millions of Americans use menstrual products, and while manufacturers contend they are safe, most disclose little about the chemicals they contain. Now, amid calls for more disclosure and research into the health effects of these products, some states require more transparency.

XTalks
SEPTEMBER 19, 2023
The US Food and Drug Administration (FDA) has issued warning letters to eight companies manufacturing or marketing unapproved eye products that violate federal law. The FDA said the warning letters are part of the agency’s ongoing effort to protect Americans from potentially harmful ophthalmic products.

STAT News
FEBRUARY 3, 2023
The dietary supplement industry has been begging for federal oversight of products containing cannabidiol (CBD), a non-psychoactive compound found in the cannabis plant that is being incorporated into a bewildering range of oils, tinctures, edibles, and other products. Read the rest…

Advarra
OCTOBER 26, 2023
Ensuring the safe and secure transport of investigational products (IP) is a core part of biosafety. Controlled Environment and IP Containment The potential for a release, and the risk associated with a genetically engineered IP, are part of the IBC’s assessment purview under National Institutes of Health (NIH) Guidelines.

XTalks
AUGUST 27, 2021
This technique results in the same taste and texture as dairy cheese, is a sustainable product and reduces greenhouse gases by being animal-free. In the past, General Mills hasn’t developed its own stand-alone plant-based brand but has launched some vegan products within existing brands. What is an Animal-Free Product?

BioTech 365
NOVEMBER 30, 2020
Lygos and High Beauty Sign Agreement to Co-Develop and Commercialize Cosmetic Products Containing Rare Cannabinoids Lygos and High Beauty Sign Agreement to Co-Develop and Commercialize Cosmetic Products Containing Rare Cannabinoids Co-branded High & Bye Refining Oil and Clearing Gel to … Continue reading →

FDA Law Blog
MARCH 21, 2024
Instead, many of these actions are essentially claim breach of warranty, in which consumers allege that they would not have purchased products at issue if the presence of PFAS had been more clearly noticed. Those claims are often triggers for plaintiff’s litigation if used with products that contain PFAS. By John W.M.

Medical Xpress
JANUARY 26, 2023
A study by scientists at Columbia University Mailman School of Public Health and Earthjustice has found that most children in the United States use makeup and body products that may contain carcinogens and other toxic chemicals.

Pharmaceutical Technology
DECEMBER 11, 2023
Thousands of over-the-counter (OTC) and prescription healthcare products contain antimicrobial agents. Here’s what you need to know when working with these ingredients.

BioTech 365
SEPTEMBER 10, 2020
| Añadir a lista de seguimiento Biotech365 : MERCK MILLIPORE ANTISTATIC GROUNDING DEVICE FOR SOLVENT CONTAINERS BioMarketplace You want to propose your products or a Biotech … Continue reading →
Druggist
OCTOBER 21, 2020
The main products used for toenail fungus treatment are available in most pharmacies, supermarkets and perhaps with the best range of products found online. Topical products, which are applied directly to the infected area, are the main products used as toenail fungus treatment.

Roots Analysis
APRIL 15, 2024
The production of biologics involves high degree of fragility and sensitivity involving the need for complex manufacturing capabilities, such as fermentation, aseptic fill finish , adequate storage with controlled temperatures and analytical testing to offer quality products.

Fierce Pharma
SEPTEMBER 13, 2023
For decades, hundreds of oral decongestant products containing phenylephrine have been available in the United States over the counter. | For decades, hundreds of oral decongestant products containing phenylephrine have been available in the United States over the counter.

Camargo
MARCH 25, 2021
The Camargo Blog is publishing a four-part blog series highlighting those designation programs available specifically for products with rare disease indications : Orphan Drug Designation (ODD), Rare Pediatric Disease Designation (RPDD), and Humanitarian Use Device (HUD) designation.

XTalks
FEBRUARY 21, 2022
BioNTech announced its new vaccine manufacturing approach in Africa where the company will ship modular factories, called the BioNTainer, to the continent to enable domestic production of the vaccines to help increase supplies. Related: BioNTech to Build First mRNA Vaccine Production Plant in Africa. Photo source: BioNTech.

The Pharma Data
SEPTEMBER 24, 2020
Some supplements billed as ‘smart drugs’ may contain unapproved drugs in potentially dangerous combinations and doses, according to a new study. The team looked through the National Institute of Health’s Dietary Supplement Label Database and the Natural Medicines Database for products containing unapproved drugs. Conor Kavanagh.

XTalks
FEBRUARY 25, 2022
EverGrain Ingredients, a barley protein and fiber solutions company, recently received certification from the Upcycled Food Association (UFA) for its entire portfolio of products. The brand saw a unique opportunity to extract the excess barley to meet the global demand for sustainable, plant-based products. Barley Milk.

Pharmaceutical Technology
SEPTEMBER 15, 2022
Research by GlobalData (Contract Small Molecule API Manufacturing Industry by the Numbers – 2021 Edition) shows that containment facilities are in high demand as the use of cytotoxic drugs continues to grow. Adding to these factors is that oncology accounts for the majority of marketed, contained drugs. Containment challenges.

Scienmag
DECEMBER 10, 2021
Use of certain personal care products during pregnancy may impact maternal hormone levels, according to a new Rutgers study. Personal care and beauty products contain several ingredients that often include a wide range of endocrine-disrupting chemicals like phthalates, parabens, phenols, parabens and toxic metals.

Camargo
FEBRUARY 16, 2021
The Camargo Blog is publishing a four-part blog series highlighting those designation programs available specifically for products with rare disease indications: Orphan Drug Designation (ODD), Rare Pediatric Disease Designation (RPDD), and Humanitarian Use Device (HUD) designation. Definition of a Rare Disease or Condition.

Advarra
AUGUST 31, 2022
However, when preparing an investigational product (IP) in a drug room or pharmacy, you need to recap the syringe and transport it to the clinic for injection. Tools or special sharp containers to remove the needle and replace with a fresh and already capped needle. The container can be a rigid plastic container (e.g.,

Pharmaceutical Commerce
JULY 11, 2023
New CSafe product, expected early next year, features a -20°C setpoint.

XTalks
JANUARY 26, 2023
While the requirement that sesame be listed as an allergen came into effect at the start of 2023, consumers may still find food products for sale that don’t list it on the label since they were produced prior to January 1st. Identifying which foods contain sesame is an important step in food safety and health care as an estimated 0.2
Scienmag
DECEMBER 16, 2021
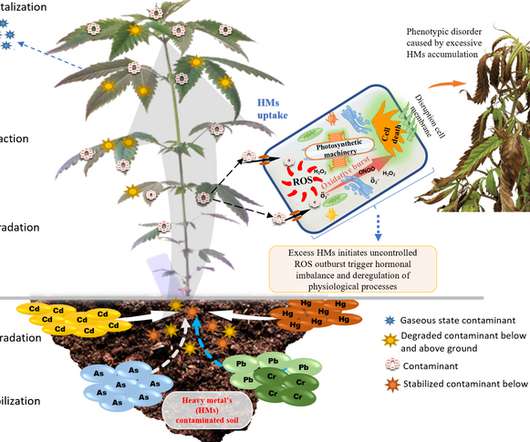
Cannabis plants — which are used to produce industrial hemp, medical marijuana and cannabidiol (CBD) oil, among other products — have an inherent ability to absorb heavy metals from the soil, making them useful for remediating contaminated sites. UNIVERSITY PARK, Pa.

Druggist
JANUARY 15, 2021
Firstly, I will list some common combination of drugs, which contain paracetamol, which may be used if standard paracetamol tablets or capsules. Combination products containing paracetamol. Ibuprofen: brands and products. Ibuprofen capsules are usually sold as a branded product. Forms of paracetamol . £2.92.

Camargo
MAY 19, 2021
The Camargo Blog has published a four-part blog series highlighting those designation programs available specifically for products with rare disease indications. The HUD designation program is designed for medical devices and is similar to the Orphan Drug Designation (ODD) program for drugs and biological products.

Sciensano
APRIL 18, 2023
It is characterisez by the purchase, transport and storage of food using its own containers. Food contact materials ( FCM ) are materials and items specifically designed to come into contact with food during its production, processing, storage, preparation or serving. But is this trend safe? Participate in the survey!

NPR Health - Shots
NOVEMBER 28, 2022
The bottom base of some Green Sprouts products can break off, exposing a solder dot that contains the toxic metal, a federal regulator said. Consumer Product Safety Commission) No injuries have been reported from such incidents. Image credit: U.S.

Pharmaceutical Technology
MAY 25, 2023
Clover Biopharmaceuticals has established a commercial collaboration with Keyuan Xinhai (Beijing) Medical Products Trading (Kyuan Trade) for the launch of its quadrivalent seasonal influenza vaccine, AdimFlu-S (QIS), in China. The vaccine contains haemagglutinin from four influenza virus strains, two A and two B.

Pharmaceutical Technology
SEPTEMBER 15, 2022
Packaging plays a vital role in maintaining the quality, safety, user-friendliness and marketability of drugs and other pharmaceutical products. The download contains detailed information on commercial pharmaceutical packaging contractors, and their product and service lines, alongside contact details to aid your hiring decision.

Pharmaceutical Technology
SEPTEMBER 6, 2022
Hospitals, clinics, pharmacies, wholesalers of medical products and other health centres are catered by pharmaceutical logistics that include handling activities such as procurement, warehousing, and inventory management, as well as transportation, and management of the supply chain for diverse products.
Pharma Mirror
SEPTEMBER 1, 2023
A key option offered with the company’s automated cleaning and disinfecting wash systems, the custom wash rack services were developed to help pharmaceutical and nutritional product manufacturers ensure complete cleaning and disinfection of laboratory glassware, tableting tools, containers, valves, machine parts, and other supplies and equipment (..)

BioSpace
SEPTEMBER 19, 2023
Eli Lilly has filed lawsuits in several states seeking to prevent unauthorized selling of products that claim to contain tirzepatide, the active ingredient in its blockbuster type 2 diabetes drug Mounjaro.

Pharmaceutical Technology
OCTOBER 6, 2022
According to Adam Bradbury, GlobalData PharmSource analyst “[…] evidence indicates that containment facilities are in high demand and will be increasingly so in future as the oncology pipeline and the use of cytotoxic drugs continues to grow.” The processes and subsequent installation must be adapted to the product,” says Housh.

Pharma in Brief
AUGUST 5, 2020
s ( Gilead ) product DESCOVY as the Canadian Reference Product. DESCOVY contains a combination of tenofovir alafenamide ( TAF ) hemifumarate and emtricitabine. The Minister determined that TAF is a new chemical entity first approved in another Gilead drug product GENVOYA. Background. Minister of Health’s Decision.

Pharmaceutical Technology
MARCH 10, 2023
Freeze drying is a crucial technique to prolong the shelf life of pharmaceutical products. Also known as lyophilisation, the technique stabilises and preserves sensitive products such as biologics, and diagnostic kits, in a permanently storable state. The process eliminates the disadvantages of conventional drying methods or freezing.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content