Phesi’s AI-driven Trial Accelerator platform contains over 100 million patients
Pharma Times
FEBRUARY 12, 2024
The platform delivers digitalised patient data to improve clinical trials and development
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharma Times
FEBRUARY 12, 2024
The platform delivers digitalised patient data to improve clinical trials and development

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 7, 2022
With an aim to improve the quality of drugs sold in the country, the Union health and family welfare ministry has released 9th edition of Indian Pharmacopoeia (IP) 2022 containing 92 new monographs for drugs, 12 new general chapters, 1,245 monographs for formulations, 930 monographs for active pharmaceutical ingredients (APIs) as well as dissolution (..)
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 17, 2024
A new drug called kush is wreaking havoc in west Africa, particularly in Sierra Leone where it is estimated to kill around a dozen people each week and hospitalise thousands. The drug, taken mostly by men aged 18 to 25, causes people to fall asleep while walking, to fall over, to bang their heads against […]

Outsourcing Pharma
FEBRUARY 6, 2024
Phesiâs artificial intelligence (AI) driven platform, Trial Accelerator, has reached a milestone and now contains global data from more than 100 million patients.
Drug Discovery World
APRIL 4, 2024
EnteroBiotix, a Glasgow, UK based biotechnology company, has dosed the first patient in a clinical trial designed to evaluate EBX-102-02 for the treatment of irritable bowel syndrome (IBS). The trial will be delivered in partnership with the Functional Gut Clinic, a leading IBS diagnosis and treatment centre.

Drug Discovery World
AUGUST 4, 2022
It may seem counterintuitive to spend time and money on planning for containment and delivery systems for a drug in the earliest stages of discovery when the chances of that molecule making it to market are still quite low. But waiting too long to assess containment and delivery carries its own risks. Testing compatibility.

Medical Xpress
APRIL 27, 2023
The role of sugars in public health continues to be urgently debated among nutrition scientists and health professionals—yet the science behind the effects of various fructose-containing sugars (e.g., sucrose/table sugar, high-fructose corn syrup, fructose/fruit sugar) on overweight and obesity has been unclear.

Rethinking Clinical Trials
SEPTEMBER 13, 2023
The DEVICE trial hypothesized that the use of a video laryngoscope will increase the incidence of successful intubation on the first attempt. The trial operated under an IRB waiver of informed consent with a patient information sheet. Discussion Themes -Why did the RSI trial follow a different path – not a waiver of consent?

Outsourcing Pharma
FEBRUARY 6, 2024
Phesiâs artificial intelligence (AI) driven platform, Trial Accelerator, has reached a milestone and now contains global data from more than 100 million patients.

Rethinking Clinical Trials
OCTOBER 2, 2023
The tool kit is intended for research teams conducting pragmatic clinical trials, including those participating in the NIH Pragmatic Trials Collaboratory’s Demonstration Projects. For more information, see the Patient-Reported Outcomes chapter of the Living Textbook.

XTalks
MAY 4, 2023
When a person with celiac disease eats something that contains gluten, their immune system attacks their small intestine, damaging the lining and interfering with the absorption of nutrients from food. However, there are ongoing clinical trials for celiac disease to investigate potential new treatments.
AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 21, 2024
Few would be left untouched by the pathogen were it to gain hold, causing healthcare systems to crumble and economies to collapse as the world once again tried to contain a force of nature. […] With a fatality rate 20 times that of COVID-19, and no vaccine, Disease X could swiftly bring humanity to its knees.

pharmaphorum
JANUARY 14, 2022
The European Commission, EMA and national regulators within the EU have launched an initiative to change the way clinical trials are designed and run in order to position the bloc as an international “focal point” for clinical research.
Drug Discovery World
OCTOBER 31, 2023
UK regulatory authorities have approved the first trial of a gene therapy for young children with Hunter syndrome. Following successful engraftment of modified HSCs in the bone marrow, these cells start to produce daughter blood cells which contain the IDS gene and enzyme which are distributed throughout the body, including the brain.

pharmaphorum
DECEMBER 4, 2022
Clinical trials of a wireless brain chip developed by Elon Muck’s Neuralink company will be tested in human volunteers within the next six months – and Musk himself says he intends to have one implanted for a future demo event. The post Elon Musk’s Neuralink brain interface chip set for human trials appeared first on. link] (12/12).

Pharma Times
AUGUST 4, 2021
Sativex is a complex botanical formulation that contains the principal cannabinoids THC and CBD
Cloudbyz
JUNE 16, 2023
Clinical trials are crucial for advancing medical research and developing innovative treatments. Effective clinical trial data archiving is essential to ensure data integrity, regulatory compliance, and seamless access. Data Complexity: Clinical trial data often comes in diverse formats, such as text, images, audio, and video.

VirTrial
NOVEMBER 29, 2023
Clinical trials demand a standardized approach to data collection, especially when it comes to patient-reported outcomes (PRO). In the ever-evolving landscape of mobile technologies, smartphones play a pivotal role in gathering crucial information from clinical trial participants. The mean usability score of 3.7/5

XTalks
NOVEMBER 29, 2022
Xtalks interviewed Devon Adams to learn more about his work related to decentralized oncology trials as a senior analyst for legislative policy specific to clinical trials at the ACS CAN. Read on to learn more! Over 1,100 cancer patients and cancer survivors responded.

Scienmag
JUNE 15, 2022
In a pilot study, researchers in ACS’ Journal of Agricultural and Food Chemistry report that compared to their pre-trial microbiome, men who drank either one alcoholic or non-alcoholic lager daily had […]. Non-alcoholic beers have become wildly popular recently, but are these drinks also healthful?

XTalks
APRIL 24, 2023
Rare disease clinical trials are complex due to the additional scientific, medical, operational and regulatory requirements of newly emerging advanced therapies, such as gene therapy,” says Dr. Terence Eagleton, MB BS, Senior Medical Director at the global clinical research organization (CRO) Medpace. Reference: Chung DC, et al.

CTTI (Clinical Trials Transformation Initiative)
JANUARY 10, 2024
Timely, accurate, and complete registration and reporting of summary results information for applicable clinical trials on ClinicalTrials.gov allows access to current research and evidence for all partners in the clinical trials enterprise, including patients, providers, sponsors and investigators, regulators, payers, and health system leaders.

pharmaphorum
NOVEMBER 28, 2021
An investigator-led trial of Jazz Pharma’s cannabis extract-based drug Sativex in glioblastoma – an aggressive form of brain cancer – will get underway in the UK next year. The post Trial of Jazz’ cannabis drug in glioblastoma will start next year appeared first on.
AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 17, 2023
The pill, called Opill – the brand name for the tablet formulation of norgestrel – is an oral contraceptive containing only progestin hormone, which helps prevent pregnancy […]

Rethinking Clinical Trials
DECEMBER 12, 2023
It has been studied in prior contexts in a PCORI trial and a pilot inpatient trial where the intervention increased goals of care discussions from 8% to 21%. Jumpstart is a pragmatic randomized trial of Jumpstart compared to usual care. In some previous trials we have randomized at the clinician level.
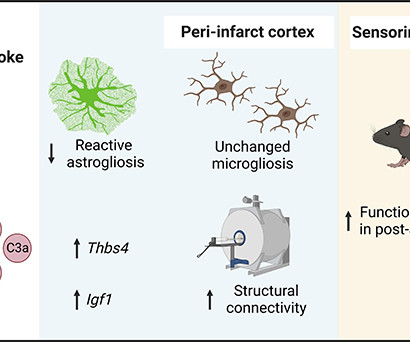
AuroBlog - Aurous Healthcare Clinical Trials blog
JUNE 22, 2023
Scientists have demonstrated how nasal drops containing a particular molecule can help mice recover from the damaging biological consequences of a stroke – and the hope is that the treatment could eventually be transferred to humans. link] Crucially, the treatment isn’t applied straight away but is initiated seven days after the stroke.

Cloudbyz
APRIL 9, 2023
Clinical trial management involves various activities and processes that can have a significant impact on the environment and contribute to sustainability issues. Clinical trials, in particular, have a significant impact on the environment, and it is essential to address this impact to ensure a sustainable future.
pharmaphorum
SEPTEMBER 14, 2020
AstraZeneca has resumed UK trials for its coronavirus vaccine, after the country’s medicines regulator gave the all-clear following a safety scare. A UK safety committee has concluded its investigations and recommended to the country’s Medicines and Healthcare products Regulatory Agency (MHRA) that trials are safe to resume.
Cloudbyz
SEPTEMBER 16, 2023
Addressing data privacy and data protection concerns when implementing ChatGPT in clinical trial operations management is crucial to maintain compliance with regulations, safeguard sensitive patient information, and build trust among stakeholders.
Pharmaceutical Technology
MAY 11, 2023
The vaccine, developed by BioNTech, led to half of the patients with pancreatic cancer in the Phase I trial remaining cancer-free 18 months later. In the study, 16 patients were treated with a vaccine called autogene cevumeran that contained a maximum of 20 neoantigens, alongside Roche’s anti-PD-L1 immunotherapy Tecentriq (atezolizumab).
Drug Discovery World
AUGUST 9, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted Aleta Biotherapeutics a clinical trial authorisation (CTA) to evaluate biologic ALETA-001 in a Phase I/II clinical trial in patients with B-cell malignancies who are relapsed/refractory to CD19 CAR T cell therapy.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 10, 2022
This means that if someone drinks 50 ml of 40 percent spirits, which contains […]. It is marketed by Swedish firm Myrkl as “the pre-drinking pill that works” The pill is said to break down up to 70 percent of alcohol after 60 minutes.

Pharmaceutical Technology
OCTOBER 31, 2022
After decades of setbacks, the respiratory syncytial virus (RSV) vaccine field has bounced back with positive Phase III trial results in older adults. However, recent data from GSK’s sub-unit vaccine, GSK-3844766A, have pushed the candidate to the top, with the highest efficacy demonstrated in a pivotal trial to date.

Worldwide Clinical Trials
FEBRUARY 21, 2024
Regardless of the formulation, the entire dose must be administered to each subject, and the dosing containers must be checked for residual radioactivity. The post Why Proactive AME Studies are Critical to Accelerating Your Approval Journey appeared first on Worldwide Clinical Trials.
pharmaphorum
AUGUST 6, 2021
Psychiatric disorders in particular represent a trial area with significantly high drop-out rates and poor patient recruitment. Despite this, new strategies devised over the last five years are beginning to reverse the trend, and both reduce stigma and increase awareness to funnel new patients into these important trials. .
Drug Discovery World
JANUARY 6, 2023
It contains multiple long peptides spanning the most immunogenic regions of the PRAME protein. . In the two-part, open label Phase Ib/II trial, ISA103 will be tested in combination with standard of care checkpoint blocker immunotherapies. A total of 90 patients will be enrolled in the trial. .
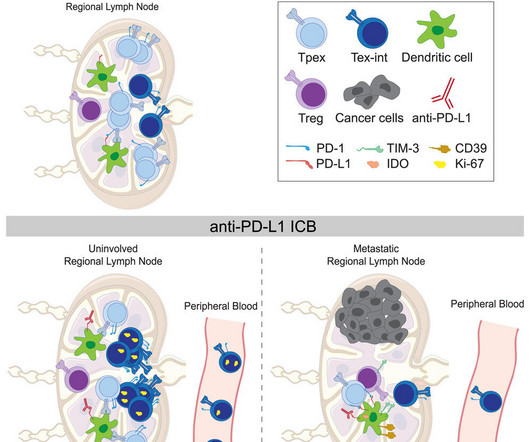
Medical Xpress
MARCH 20, 2023
Cancer treatment routinely involves taking out lymph nodes near the tumor in case they contain metastatic cancer cells. But new findings from a clinical trial by researchers at UC San Francisco and Gladstone Institutes shows that immunotherapy can activate tumor-fighting T cells in nearby lymph nodes.

Pfizer
FEBRUARY 16, 2023
These study participants, representing approximately half of the total recruited participants in the trial, are being discontinued following violations of Good Clinical Practice (GCP) at certain clinical trial sites run by a third-party clinical trial site operator.
pharmaphorum
NOVEMBER 16, 2022
“The aim is to identify a GSK3036656-containing regimen with sufficient tolerability, efficacy, and short enough duration to progress to phase 3 with a high probability of success,” added GSK. The post As resistant tuberculosis rises, GSK says trial backs novel antibiotic appeared first on.

AuroBlog - Aurous Healthcare Clinical Trials blog
JUNE 12, 2023
The Multidisciplinary Committee (MDC) of Experts has recommended the prices of various fixed dose combinations (FDCs) containing dapagliflozin, sitagliptin, vildagliptin, pioglitazone, among others in tune with the recent amendments made by the Department of Pharmaceuticals (DoP) in the Drugs (Prices Control) Order (DPCO), 2013 related to fixing prices (..)

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 2, 2023
The 9-valent HPV vaccine—Gardasil-9—helps in reducing the disease burden and cancers caused by the HPV types contained in […] Gardasil-9 is India’s first gender-neutral HPV vaccine.

Pharmaceutical Technology
JUNE 5, 2023
Led by the investment company Temasek, the round will also be used to improve the company’s commercial readiness as its lead radiopharmaceutical candidate ITM-11 is being studied in Phase III trials in gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Other investors included BlackRock, the Qatar Investment Authority, and Nextech.

XTalks
MAY 7, 2021
Novavax published the latest results from a Phase IIb clinical trial conducted in South Africa evaluating the safety and efficacy of NVX-CoV2373, appearing in the NEJM ’s May 6, 2021 issue. Earlier results from a more complete analysis of trial data shared in March confirmed a high level efficacy of 86.3 1.351 variant.

Pharmaceutical Technology
APRIL 28, 2023
It has also been approved by the FDA for otitis media prevention in infants and children aged between six weeks and five years caused by the original seven serotypes contained in PREVNAR. The latest approval is based on data obtained from the Phase II and Phase III trials of PREVNAR 20 for paediatric indication.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content