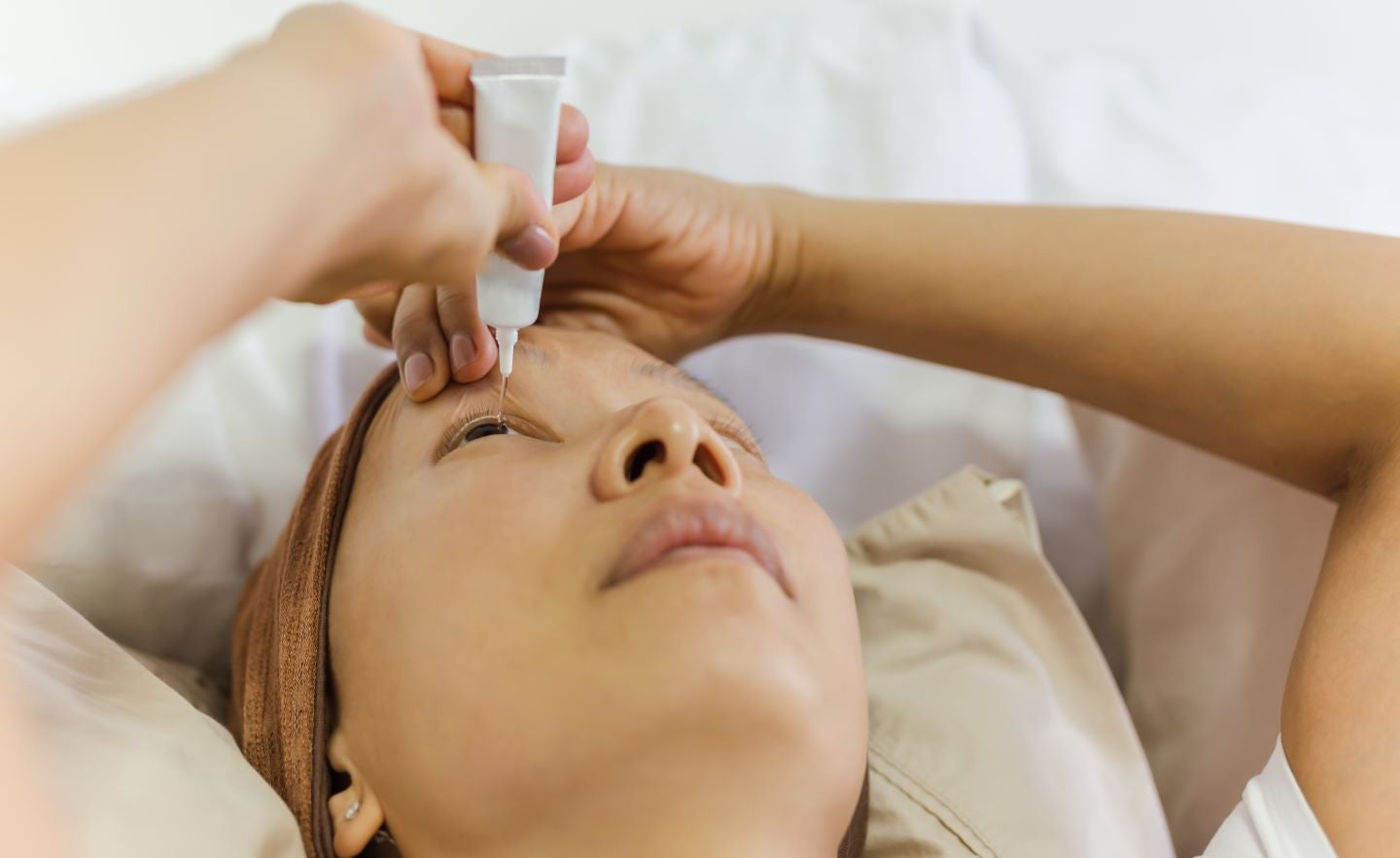
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has issued a recall of batches of carbomer-containing lubricating eye gels due to potential microbial contamination.
Eye gels containing carbomer provide relief from dry eye symptoms.
The recalled gels are sold under the brand names Aacarb, Aacomer and Puroptics.
Issuing precautionary safety advice, the regulator warned patients to stop using the products and return them to their retailer.
Any user experiencing eye infection symptoms such as reduced vision or a red eye with pain should contact their healthcare provider.
See Also:
Investigations are underway to ascertain if there are links between identified infections and usage of the recalled products.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataMHRA Chief Safety Officer Alison Cave stated: “Whilst the risk to users is low, we are taking precautionary action.
“If you have been using these gels, and if your eye symptoms worsen or you are worried about your health, please contact your healthcare professional, and tell them you have been using an eye gel that has been recalled.
“Retailers should, where possible, contact patients who have been dispensed any of the affected batches and ask them to return the product.”
The UK Health Security Agency has recommended that people with cystic fibrosis, those who are severely immunocompromised, patients with specific risk factors and individuals awaiting lung transplants avoid usage of all carbomer-containing lubricating eye gels until further notice.