Clinical trials and development services
Pharmaceutical Technology
APRIL 4, 2024
Download our comprehensive list of leading specialist companies offering clinical trials and development services for free today.

Pharmaceutical Technology
APRIL 4, 2024
Download our comprehensive list of leading specialist companies offering clinical trials and development services for free today.
Worldwide Clinical Trials
APRIL 3, 2024
As with other targeted therapies, radiopharmaceutical development requires unique and specialized strategies to ensure successful execution. Are you aware of the challenges you must address for a successful radiopharmaceutical trial? These include ensuring informed consent, particularly around the risks and benefits of participation.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
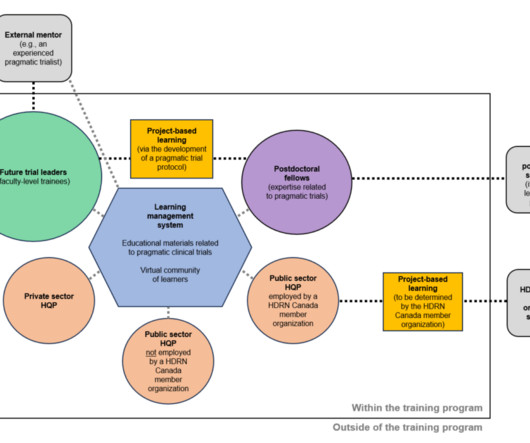
Rethinking Clinical Trials
DECEMBER 5, 2023
Health Data Research Network (HDRN) Canada is now accepting applications for its Pragmatic Trials Training Program. There will be an emphasis on experiential learning for future trial leaders and postdoctoral fellows in the program through the development of a pragmatic trial protocol.

Pharmaceutical Technology
JUNE 15, 2023
But before pharmaceutical companies can go to market with a breakthrough drug, they need to ensure safety and efficacy through clinical trials. Pharma R&D teams are solving this problem by leveraging the power of artificial intelligence (AI) in clinical trials to save time and money. Food and Drug Administration approval.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies.

Rethinking Clinical Trials
MARCH 28, 2024
Rosa Gonzalez-Guarda The NIH Pragmatic Trials Collaboratory’s Health Equity Core developed a written aid to offer guidance on inclusive language and terms to use when referring to specific people, groups, and communities. View the Equitable Language Cheat Sheet.

Bio Pharma Dive
JANUARY 10, 2023
Moderna’s Stéphane Bancel and Pfizer’s Albert Bourla hinted Monday at greater dealmaking to come for their companies, while Gilead gave investors an update on an important cancer drug trial.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials.
Let's personalize your content