
In October 2023, Katalin Karikó, PhD and Drew Weissman, PhD won the Nobel Prize in Physiology or Medicine for their work with messenger ribonucleic acid (mRNA), which laid the foundation for COVID-19 mRNA vaccines.
mRNA therapies and vaccines have revolutionised the drug development space in the last few years thanks to breakthroughs in advanced research. Now, only a few years after the first mRNA vaccine was authorised, the mRNA approach may soon be trumped by circular RNA (circRNA).
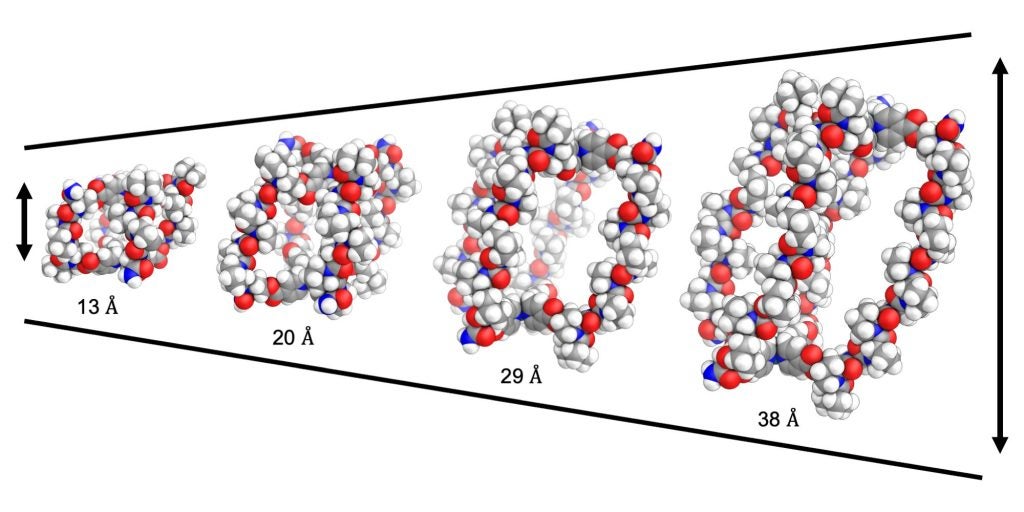
CircRNA is a type of single-stranded RNA that, unlike linear RNA, forms a covalently closed continuous loop. In circular RNA, the 3’ and 5’ ends that are normally present in an RNA molecule are joined together.
mRNA vaccines will be key in preventing and treating diseases in the future, but expensive manufacturing, requirements for cold-chain storage, and difficulties in transporting make their use complicated. In such circumstances, circRNA could step in and take its place.
Alex Wesselhoeft, PhD, director of RNA Therapeutics at the Gene and Cell Therapy Institute, Mass General Brigham, says it is still early days with circRNA, but based on preclinical research, these candidates could be even better than mRNA therapies.
“It took mRNA 30 years to become what it is. In terms of just what the technology can do however, [circRNA] could be mRNA 2.0,” Wesselhoeft explains.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataCharacteristics of circRNA could make it superior
The closed loop structure of circRNA could make more stable, longer lasting vaccine candidates compared to linear-based mRNA. Additionally, these advantages could also mean that less of the genetic material is required with circRNA, meaning that more vaccines could be effective as single doses and patients could suffer fewer adverse events since lower quantities of the active ingredient would be needed.
Nikolaus Rajewsky, PhD, who runs the circRNA lab at the Max Delbrück Centre for Molecular Medicine in Berlin, says a number of big companies are building a pipeline of circRNA candidates. At the same time, he says, “We also understand very little still about circRNAs despite having had many papers published. The problem here is the bigger insights are always difficult.”
Erik Wiklund, CEO, Circio Holding, a biotech investigating circRNA as therapeutics, says it will take some time but he is also hopeful circRNA could be the next big breakthrough in the RNA field. Circio is exploring the possibilities of a universal flu vaccination; however, as most of the big pharma companies investigating circRNA are focusing on its potential in vaccines, Wiklund said that the team at Circio decided to focus most of its efforts on the therapeutics field.
“We believe that we can make a massive improvement on adeno-associated virus (AAV)-based gene therapy,” Wiklund explains. “Almost all gene therapies approved today are based on the AAV virus but the big caveat is that you need to give really high doses when you express the gene of interest using mRNA.” Moreover, safety problems like liver toxicities, and in some instances serious adverse events and death, are also a challenge. “Using our circRNA surface technology, we anticipate that we can increase the protein output by maybe up to 100-fold,” Wiklund says.
With circRNA, the candidate will contain one hundredth the amount of AAV of current therapies, therefore it is likely to have a better safety profile as well as becoming easier and cheaper to manufacture. AAVs are unable to carry a great deal of genetic material. Due to circRNA being longer-lasting due to its closed loop structure, less is required to be as effective, resulting in a reduced dose of AAV to carry the same dose capability.
Wiklund adds the company is focusing on its lead therapeutic candidate treating patients with alpha-1 antitrypsin deficiency.
Although therapies using circRNA will benefit heavily from the reduced dosing needs, it is not likely that these could be developed as a one-and-done type therapy such as Vertex Pharmaceuticals’ Casgevy (exagamglogene autotemcel), and multiple infusions through a patient’s life may be needed, he adds. Casgevy is a medicine used to treat the blood disorders beta thalassaemia and sickle cell disease in patients 12 years and older. It is the first clustered regularly interspaced short palindromic repeats (CRISPR) therapy to receive approval in the US, UK, and Europe.
Potential as a biomarker for neurodegenerative disease
Amid the great deal of preclinical research being conducted in circRNA, one significant discovery over the past decade has been regarding its high presence in neurons.
Research from Rajewsky’s lab suggests circRNA could be a potential biomarker for neurodegenerative diseases. Moreover, circRNAs are also found in the blood and could also be effective as a biomarker there, Rajewsky adds.
If circRNA can be utilised as a biomarker at earlier stage of disease, potentially before symptoms develop in some diseases such as Alzheimer’s or Parkinson’s, it may become easier for drug developers to develop therapies that can better delay disease development, which would have a huge impact on healthcare.
“The discovery that circRNA plays an important role in age-associated diseases, including neurodegenerative disease, cancer and diabetes, among others, allows for this structure to be utilised as a potential disease biomarker,” says Anaelle Tannen, Healthcare Analyst at GlobalData. “This could serve as a revolutionary change in preventing old-age disease, reducing the health and socioeconomic burden on society.”
Despite their discovery nearly 12 years ago, circRNA biomarkers are still not being heavily investigated, Rajewsky adds. Still, he goes on to say that circRNA biomarkers are likely to be approved before any vaccines or therapeutics.
Early trials will focus on safety
These benefits have so far only been observed in preclinical research, so until first-in-human studies are conducted, it is difficult to say whether these will translate.
“Experimenting in a clinical context is required in order to assess whether this therapeutic format will be safe and efficacious in the real world,” explains Tannen. “Research will also help in understanding the physiological and pathological mechanisms of circRNAs, and how these may benefit or impede human life.”
Trial designs may be amended due to the individual characteristics of these candidates, but it is unlikely these trials will be significantly different from traditional trials, says Rajewsky.
One of the most likely changes in trial design will be that patients will be monitored for longer to ensure safety due to the longer acting feature of these candidates. There will also be quite a bit of investigation with dosing levels until there is a better understand of quite how efficacious these candidates can be.
“I think that the first few Phase I trials will be used to guide development of the [circRNA] platform. It’s not like a small molecule where you have one shot and then you’re done. These are codable technologies that you can continually improve and develop,” says Wesselhoeft, who expects to see some clinical results from drugs using this approach in the next two to five years.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.