ABOUT AUTHORES
AKASH GUPTA M.Pharm (pharmaceutics)
akashav88@gmail.com
Kota college of pharmacy,ranpur, kota - 325003
ABSTRACT
The purpose of writing this review on floating drug delivery systems (FDDS) was to The recent developments of FDDS including the physiological and formulation variables affecting gastric retention, approaches to design single-unit and multiple-unit floating systems, and their classification and formulation aspects are covered in detail. Prolonged gastric retention improves bioavalibility, reduce drug waste, and improve solubility for drugs that are less soluble in a high ph environment. It has applications also for local drug delivery to the stomach and proximal small intestines.
INTRODUCTION
Floating tablet:- floating tablet is a gastro rentative doasage form increase the retention time of drug in git and plenty of advantage over the conventional tablets. The most important application of with helicobacter pylori.The floating tablet is that provide a new possibility of treating the stomach infected.
Floating drug delivery system:-
Oral administration is the most convenient mode of drug delivery and is associated with superior patient compliance as compared to other modes of drug intake. However, oral administration has only limited use for important drugs, from various pharmacological categories, that have poor oral bioavailability due to incomplete absorption and/or degradation in the gastrointestinal (GI) tract. Some of these drugs are characterized by a narrow absorption window (NAW) at the upper part of the gastrointestinal.
Gastric emptying of dosage forms is an extremely variable process and ability to prolong and control emptying time is a valuable asset for dosage forms, which reside in the stomach for a longer period of time than conventional dosage forms. Several difficulties are faced in designing controlled release systems for better absorption and enhanced bioavailability. One of such difficulties is the inability to confine the dosage form in the desired area of the gastrointestinal tract. Drug absorption from the gastrointestinal tract is a complex procedure and is subject to many variables. It is widely acknowledged that the extent of gastrointestinal tract drug absorption is related to contact time with the small intestinal mucosa . Thus small intestinal transit time is an important parameter for drugs that are incompletely absorbed.
Gastro Retentive Drug Delivery Devices :-
These are primarily controlled release drug delivery systems, which gets retained for longer period of time in stomach, thus helping in absorption of drug for the intended duration of time, which in turn improves bioavailability by reducing drug wastage, and improving solubility of drugs that are less soluble at high pH environment . It also helps in achieving local delivery of drug in the stomach and proximal small intestine. G.R.D.D devices can be useful for the spatial and temporal delivery of many drugs.
Ideal candidates for gastro retentive drug delivery systems:
• Drug which act locally in the stomach.
• Drugs which get primarily absorbed in the stomach.
• Drugs which are poorly soluble at alkaline pH.
• Drugs with a narrow therapeutic window of absorption.
• Drugs which are absorbed rapidly from GI tract.
• Drugs that degrade in the colon.
Approaches to Gastric Retention
i) Mucoadhesive or bioadhesive systems:- These system permit a given dosage form to be incorporated with bioadhesive agent or mucoadhesive agents making the device to get adhered to the stomach walls, thus resisting gastric emptying.But it may result in unpredictable adherence as the mucus on the walls of the stomach is in a state of constant renewal.
ii) Floating drug delivery systems (FDDS):- The floating drug delivery systems float in the stomach after its administration. These systems are based on the following mechanisms:
• Hydrodynamically balanced systems (HBS):- These are single-unit dosage forms, containing one or more gel-forming hydrophilic polymers such as Hydroxypropyl methylcellulose (HPMC) although hydroxy ethyl cellulose (HEC), hydroxyl propyl cellulose (HPC), sodium carboxy methylcellul (NaCMC), agar, or alginic acid . The polymer is being mixed with the drug and usually administered in a gelatin capsule. The capsule gets rapidly dissolved in the gastric fluid, producing a floating mass. Drug release is getting controlled by the formation of a boundary at the surface. Continuous erosion of the surface allows water to get penetrated into the inner layers, maintaining surface hydration and helping in its buoyancy. Incorporation of fatty materials gives low-density formulations and reduced penetration of water thus reducing the erosion quickly.
iii) High density systems
Gastric contents in the G.I.T have density close to that of water. When the patient is upright high-density tablets sink to bottom of the stomach and they get entrapped in the folds of the antrum and able to withstand the peristaltic waves of the stomach .
iv) Magnetic systems
This system is based on a simple idea that the dosage form contains a small internal magnet, and a magnet is placed on the abdomen over the position of the stomach. Although these systems seem to work, the external magnet must be positioned with a degree of precision that might compromise patient compliance.
v) Superporous hydrogels
Although these are swellable systems they differ sufficiently from the conventional types to warrant separate classification, with pore size ranging 10nm10µm.Absorption of water by conventional hydrogel is a very slow process and several hours may be needed to reach an equilibrium state and during which premature evacuation of the dosage form may occur. Superporous hydrogels having an average pore size of greater than 100µm, swell to equilibrium size with in a minute, due to rapid water uptake by capillary wetting through numerous inter-connected open pores and they swell to a larger size and are intended to have sufficient mechanical strength to withstand pressure by gastric contraction. This is achieved by co-formulation of a hydrophilic particulate material.
Advantages of GRDF’s
• Enhanced bioavailability of drugs which are mainly absorbed from the upper part of the gastro Intestinal system.
• Useful for local treatment in case of stomach ulcers and lesions.
• Improving patient compliance by reducing dose.
• Enhanced therapeutic efficacy.
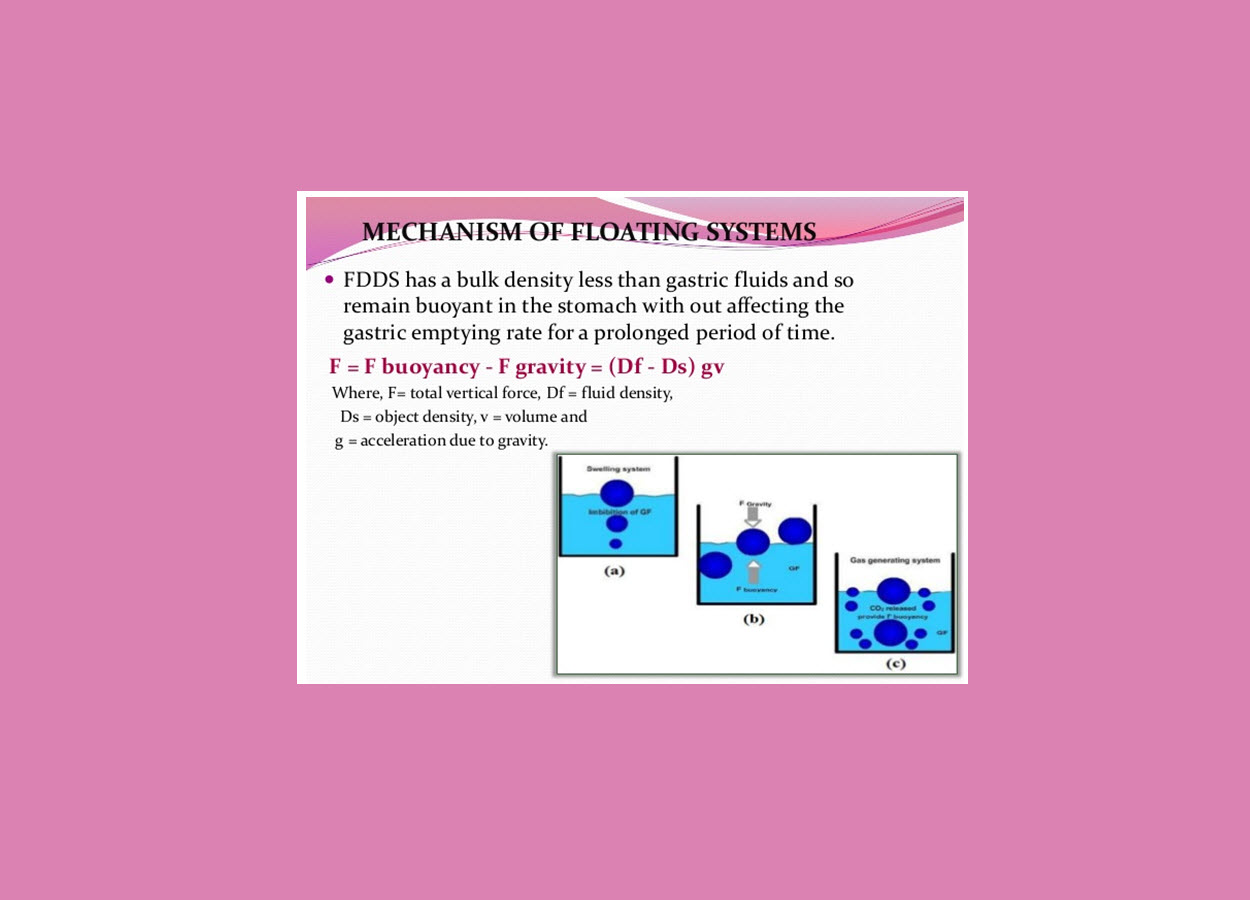
Mechanism of floating systems :
Floating drug delivery systems (FDDS) have a bulk density less than gastric fluids and so remain buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time. While the system is floating on the gastric contents, the drug is released slowly at the desired rate from the system.however ,besides a minimal gastric content needed to allow the proper achievement of the buoyancy retention principle, a minimal level of floating force (F) is also required to keep the dosage form reliably buoyant on the surface of the eal. To measure the floating force kinetics,a novel apparatus for determination of resultant weight has been reported in the literature. Te apparatus operates by measuring continuously the force equivalent to F(as a functionof time)that is required to maintain the submerged object. Te object floats better if F on the higher positive side.this appratus helps in optimizing FDDS with respect to stability and durability of floating forces produced in order to prevent the drawbacks of unforeseeable intragastric buoyancy capability variations.
The equation given in fig1
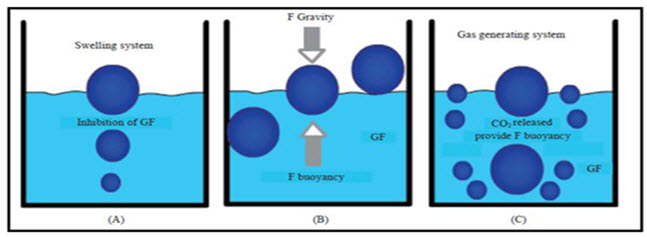
Fig-2
FORMULATION EXCIPIENTS USED IN FDDS
1.Polymers:- The following polymers used in preparations of FDDS -HPMC K4 M, Calcium alginate, Eudragit S100, Eudragit RL, Propylene foam, Eudragit RS, ethyl cellulose, poly methyl methacrylate, Methocel K4M, Polyethylene oxide, β Cyclodextrin,HPMC 4000, HPMC 100, CMC, Polyethylene glycol, polycarbonate, PVA, Polycarbonate, Sodium alginate, HPC-L, CP 934P, HPC, Eudragit S, HPMC, Metolose S.M. 100, PVP, HPC-H, HPC-M, HPMC K15, Polyox, HPMC K4, Acrylic polymer, E4 M and Carbopol
2.Inert fatty materials (5%-75%):- Edible, inert fatty material having a specific gravity of less than one can be used to decrease the hydrophilic property of formulation and hence increase buoyancy. E.g. Beeswax, fatty acids, long chain fatty alcohols, Gelucires 39/01 and 43/01.
3. Effervescent agents:- Sodium bicarbonate, citric acid, tartaric acid, Di-SGC (Di-Sodium Glycine Carbonate, CG (Citroglycine).
4. Release rate accelerants (5%-60%):- eg. lactose, mannitol.
5. Release rate retardants(5%-60%):- eg. Dicalciumphosphate,talc, magnesium stearate
6. Buoyancy increasing agents (upto80%):- eg. Ethyl cellulose
7. Low density material Polypropylene foam powder (AccurelMP 1000).
EVALUATION OF FLOATING DRUG DELIVERY SYSTEMS
1. SIZE AND SHAPE EVALUATION
The particle size and shape plays a major role in determining solubility rate of the drugs and thus potentially its bioavailability. The particle size of the formulation was determined using Sieve analysis (Jayant, Mumbai), Air elutriation (Bahco TM) analysis, Photo analysis, Optical microscope (Olympus (India) pvt.ltd), Electro résistance counting methods (Coulter counter), Sedimentation techniques, Laser diffraction methods, ultrasound attenuation spectroscopy, Air Pollution Emissions Measurements .
2. FLOATING PROPERTIES
Effect of formulation variables on the floating properties of gastric floating drug delivery system was determined by using continuous floating monitoring system and statistical experimental design.
3. SURFACE TOPOGRAPHY
The surface topography and structures were determined using scanning electron microscope (SEM, JEOL JSM – 6701 F, Japan) operated with an acceleration voltage of 10k.v, Contact angle meter, Atomic force microscopy (AFM), Contact profiliometer.
4. DETERMINATION OF MOISTURE CONTENT
The water content per se is seldom of interest. Rather, it shows whether a product intended for trade and production has standard properties such as
• Storability
• Agglomeration in the case of powders
• Microbiological stability
• Flow properties, viscosity
• Dry substance content
• Concentration or purity
• Commercial grade (compliance with quality agreements) Thus moisture content of the prepared formulations was determined by Karl fisher titration, vacuum drying, Thermo gravimetric methods, Air oven method, Moisture Meters, Freeze drying as well as by physical methods .
5. SWELLING STUDIES
Swelling studies were performed to calculate molecular parameters of swollen polymers. Swelling studies was determined by using Dissolution apparatus, optical microscopy and other sophisticated techniques which include H1NMRimaging, Confocal laser scanning microscopy (CLSM), Cryogenic scanning electron microscopy (Cryo SEM), Light scattering imaging (LSI) etc. The swelling studies by using Dissolution apparatus (USP disso-lution apparatus (usp-24) lab india disso 2000) was calculated as per the following formula . 51 Swelling ratio = Weight of wet formulation / Weight of formulations.
6. DETERMINATION OF THE DRUG CONTENT
Percentage drug content provides how much amount of the drug that was present in the formulation. It should not exceed the limits acquired by the standard monographs. Drug content was determined by using HPLC, HPTLC methods, Near infrared spectroscopy (NIRS), Micro titrimetric methods, Inductively Coupled Plasma Atomic Emission Spectrometer (ICPAES) and also by using spectroscopy techniques (Elico Limited, Hyderabad) .
7. PERCENTAGE ENTRAPMENT EFFICIENCY
Percentage entrapment efficiency was reliable for quantifying the phase distribution of drug in the prepared formulations. Entrapment efficiency was determined by using three methods such as Micro dialysis method, Ultra centrifugation, and pressure Ultra filtration.
8. IN-VITRO RELEASE STUDIES
In vitro release studies (USP dissolution apparatus (usp-24) lab india disso 2000) were performed to provide the amount of the drug that is released at a definite time period. Release studies were performed by using Franz diffusion cell system and synthetic membrane as well as different types of dissolution apparatus.
9. POWDER X-RAY DIFFRACTION
X-ray powder diffraction (Philips analytical, model-pw1710) is the predominant tool for the study of poly-crystalline materials and is eminently suited for the routine characterization of pharmaceutical solids. Samples were irradiated with α radiation and analyzed between 2 ºC and 60 ºC .The voltage and current used were 30KV and 30mA respectively.
10. FOURIER TRANSFORMS INFRARED ANALYSIS
Fourier transform infrared spectroscopy (FT-IR, Shimadzu, Model-RT-IR-8300) is a technique mostly used to identify organic, polymeric, and some inorganic materials as well as for functional group determination. Fourier Transform Infrared Analysis (FT-IR) measurements of pure drug, polymer and drug loaded polymer formulations were obtained on FT-IR. The pellets were prepared on KBr press under hydraulic pressure of 150kg/cm2; the spectra were scanned over the wave number range of 3600 to 400 cm-1 at the ambient temperature.
PHARMACO DYNAMIC ASPECTS OF FDDS
1. Reduced fluctuations of drug concentration
Continuous input of the drug following floating system administration produces blood drug concentrations within a narrower range compared to the immediate release dosage forms. Thus, fluctuations in drug effects are minimized and concentration dependent adverse effects that are associated with peak concentrations can be prevented. This feature is of special importance for drugs with a narrow therapeutic index. Improved selectivity in receptor activation: Minimization of fluctuations in drug concentration also makes it possible to obtain certain selectivity in the elicited pharmacological effect of drugs that activate different types of receptors at different concentrations. Reduced counter-activity of the body: In many cases, the pharmacological response, which intervenes with the natural physiologic processes, provokes a rebound activity of the body that minimizes drug activity. Slow input of the drug into the body was shown to minimize the counter activity leading to higher drug efficiency.
2. Minimized adverse activity at the colon
Retention of the drug in the GRDF at the stomach minimizes the amount of drug that reaches the colon. Thus, undesirable activities of the drug in colon may be prevented. This pharmacodynamic aspect provides the rationale for floating formulation for betalactam antibiotics that are absorbed only from the small intestine and presence in the colon leads to development of microorganisms
CONCLUSION
Drug absorption in the gastrointestinal tract is a highly variable procedure and prolonging gastric retention of the dosage form extends the time for drug absorption. FDDS promises to be a potential approach for gastric retention. Although there are number of difficulties to be worked out to achieve prolonged gastric retention, a large number of companies are focusing towards commercializing this technique.
REFERENCES :-
1. Talukder, Fassinir R. Gastro retentive delivery system: Hollow beads. Drug Dev Ind Pharm. 2004; 4: 405-412
2. Singh BN, Kim KH. Floating drug delivery system: Approach to oral controlled drug delivery via gastric retention. J Control Rel. 2000; 63: 235-259.
3. oseph NJ, Laxmi S, Jayakrishnan A. A floating type oral dosage from for piroxicam based on hollow microspheres: In vitro and in vivo evaluation in rabbits. J Control Rel. 2002; 79: 71-79.
4. Nur AO, Zhang JS. Captopril floating and/or bioadhesive tablets: Design and release kinetics. Drug Dev Ind Pharm. 2000; 26: 965-969.
5. Desai S and Bolton S. A floating controlled release drug delivery system: in vitro- in vivo evaluation. Pharm Res. 1993;10:1321-1325.
6. Vantrappen GR, Peeters TL and Janssens J. The secretory component of interdigestive migratory motor complex in man. Scand J Gastroenterol. 1979;14:663-667.
7. Wilson CG and Washington N. The stomach: its role in oral drug delivery. In: Rubinstein MH,ed. Physiological Pharmacetical: Biological Barriers to Drug Absorption. Chichester, UK:Ellis Horwood. 1989; 47-70.
8. Sing BN and Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Rel. 2000;63:235-59.
9. Sungthongjeen S, Paeratakul O, Limmatvapirat S and Puttipupathachorn S. Preparation and in-vitro evaluation of multiple-unit floating drug delivery system based on gas formation technique. Int J Pharm. 2006;324:136-43.
10. Krogel I and Bodmeier R. Development of a multifunctional matrix drug delivery system surrounded by an impermeablecylinder. J Control release. 1999;61:43-50
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE












.png)

