Springworks shares fall despite drug trial's success
Bio Pharma Dive
MAY 24, 2022
The biotech plans to seek FDA approval for its soft-tissue tumor treatment after positive study results. But investors still sent the stock down by nearly 10%.

Bio Pharma Dive
MAY 24, 2022
The biotech plans to seek FDA approval for its soft-tissue tumor treatment after positive study results. But investors still sent the stock down by nearly 10%.

XTalks
AUGUST 3, 2022
In this episode, Ayesha discussed the FDA approval of Azurity Pharmaceutical’s Zonisade (zonisamide oral suspension) as an adjunct therapy for the treatment of seizures in adults and pediatric patients 16 years of age and older with epilepsy. The drug is the first FDA approved oral suspension form of zonisamide.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Discovery World
NOVEMBER 29, 2023
“The most important benefit is that we now have an accurate model that we can use to perform virtual experiments with different types of interventions, quickly and digitally.”
Drug Discovery World
MAY 3, 2023
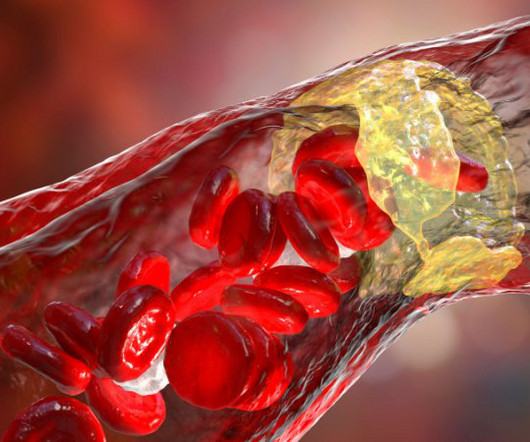
Official comments “Measuring endothelial dysfunction with the EndoPAT technology in drug trials is a cost-effective and robust method for early documentation of proof-of-concept in pulmonary, renal and vascular diseases” says Elin Rosendahl, VP Clinical Operations, Vicore Pharma.
Advarra
AUGUST 16, 2022
Orphan drugs have historically faced a number of barriers, such as limited research and development (R&D) investment due to an expected lack of profitability as well as challenges in clinical trial design and recruitment. Before 1983, only 38 orphan drugs had received U.S. Food and Drug Administration (FDA) approval.

Drug Discovery World
MARCH 7, 2023
She founded Neuroute in 2019, with the aim of empowering patients from diverse backgrounds to participate in drug trials. Other roles include Head of Biology at TopoTarget A/S, where she was responsible for the pre-clinical development of belinostat which went on to gain FDA approval to treat peripheral T-cell lymphoma.
XTalks
AUGUST 4, 2022
This started back in 1993 when the FDA established guidelines to increase diversity by gender and race/ethnicity of participants in clinical trials that were contributing to new drug approvals. An analysis of trials that supported FDA approvals of new cardiometabolic drugs from 2008 to 2017.
Let's personalize your content