Takeda secures FDA approval for colon cancer drug
Bio Pharma Dive
NOVEMBER 9, 2023
The pharma paid $400 million to license the drug from Hutchmed earlier this year in an effort to bolster its oncology business.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Bio Pharma Dive
NOVEMBER 9, 2023
The pharma paid $400 million to license the drug from Hutchmed earlier this year in an effort to bolster its oncology business.

Pharmaceutical Technology
JANUARY 9, 2023
Eisai and Biogen have received approval for their antibody Leqembi (lecanemab-irmb) , 100mg/mL injection for intravenous use, from the US Food and Drug Administration (FDA) under the Accelerated Approval Pathway to treat Alzheimer’s disease (AD).
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
AUGUST 8, 2022
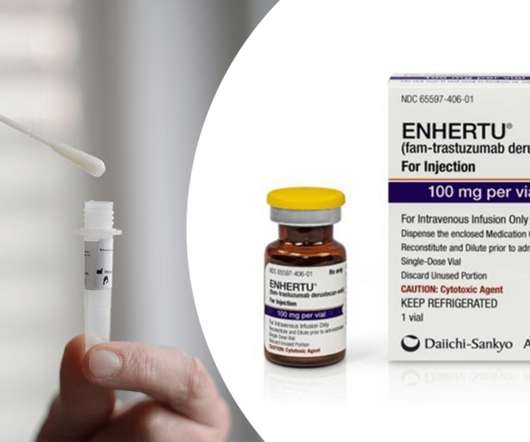
AstraZeneca and Daiichi Sankyo ’s Enhertu (trastuzumab deruxtecan) has received expanded approval from the US Food and Drug Administration (FDA) to treat adults with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer.

XTalks
APRIL 3, 2024
The US Food and Drug Administration (FDA) has given the nod to AstraZeneca’s Voydeya (danicopan) as an add-on therapy to the company’s standard-of-care C5 inhibitors Ultomiris (ravulizumab) or Soliris (eculizumab) for the treatment of extravascular hemolysis (EVH) in adults with the rare blood disease paroxysmal nocturnal hemoglobinuria (PNH).
Fierce Pharma
NOVEMBER 9, 2023
AstraZeneca licensed an oral GLP-1 agonist from China's Eccogene. Takeda won FDA approval for the Hutchmed-developed colorectal cancer drug Fruzaqla. AstraZeneca licensed an oral GLP-1 agonist from China's Eccogene. Takeda won FDA approval for Hutchmed-developed colorectal cancer drug Fruzaqla.

Pharmaceutical Technology
MAY 10, 2023
Gilead Sciences has emerged victorious in a legal battle with the US government over patents surrounding the HIV pre-exposure prophylaxis (PrEP) drugs Descovy and Truvada following a federal jury’s verdict on May 9. The FDA approved Truvada as the first PrEP treatment in July 2012, with Descovy receiving a nod for this use in October 2019.

XTalks
JULY 25, 2023
Emergent BioSolutions, a multinational specialty biopharmaceutical company headquartered in Gaithersburg, Maryland, has achieved a significant milestone with the approval of its Cyfendus (Anthrax Vaccine Adsorbed, Adjuvanted) vaccine by the US Food and Drug Administration (FDA). Overall, 66.3 on Day 64 in the study.

BioTech 365
MARCH 16, 2021
FSD Pharma Enters into License Agreement to Develop FDA approved Veterinary Drugs for the Treatment of Gastro-Intestinal Diseases in Dogs and Cats FSD Pharma Enters into License Agreement to Develop FDA approved Veterinary Drugs for the Treatment of Gastro-Intestinal Diseases … Continue reading →

XTalks
JANUARY 4, 2023
MediWound, a biopharmaceutical company focused on biotherapeutic solutions for tissue repair and regeneration, announced that the US Food and Drug Administration (FDA) approved their orphan biological product NexoBrid (anacaulase-bcdb) for the removal of eschar in adults with deep partial-thickness and/or full-thickness thermal burns.

Pharmaceutical Technology
APRIL 17, 2023
US-based development-stage biopharmaceutical firm Satsuma Pharmaceuticals is developing STS101, a unique nasal powder formulation of the anti-migraine drug dihydroergotamine mesylate, for the treatment of acute migraine. In March 2023, Satsuma submitted a new drug application for STS101 to the US Food and Drug Administration (FDA).
XTalks
AUGUST 10, 2022
In this episode, Ayesha discussed a new COVID-19 test technology that Innova Medical Group, world leader in at-home COVID-19 tests, has reached a licensing deal for with the University of Birmingham where the technology was developed. The approval makes Enhertu the first approved drug for this indication.

Pharmaceutical Commerce
DECEMBER 20, 2023
If the FDA approves the sBLA for Xolair, it would be the first drug indicated to lower allergic reactions to multiple foods after an accidental exposure.
XTalks
DECEMBER 7, 2022
Rigel Pharmaceuticals’ isocitrate dehydrogenase-1 mutant (mIDH1) targeting small molecule inhibitor Rezlidhia (olutasidenib) has won US Food and Drug Administration (FDA) approval for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML). The median time to complete remission was 1.9

XTalks
OCTOBER 22, 2021
The US Food and Drug Administration (FDA) has approved Cyltezo (adalimumab-adbm) as the first interchangeable biosimilar for Humira (adalimumab) for the treatment of several inflammatory conditions. This means AbbVie, maker of the world’s top-selling drug Humira, could be in for some stiff competition.

Drug Discovery World
FEBRUARY 23, 2024
In a breakthrough for advanced therapies, this week saw the FDA approve the first ever cell therapy for solid tumour cancers, but there were other significant developments in the cell and gene therapy space. The post This week in drug discovery (19-23 February) appeared first on Drug Discovery World (DDW).

pharmaphorum
NOVEMBER 15, 2022
Lantheus’ pipeline-building drive in radiopharma has continued with a $260 million upfront deal to license rights to two oncology candidates from POINT Biopharma. billion in milestone payments to POINT if the two radiotherapeutics get FDA approval and meet commercial objectives, plus royalties on net sales.
Pharmaceutical Technology
MARCH 27, 2023
In 2022, the US FDA disappointingly approved almost a third fewer of the most innovative drugs than it did in 2021. During 2013–2022, NME approvals were lower only in 2013 and 2016. Faced with such a disappointing approval number last year, manufacturers hope for more NMEs to enter the market in 2023.
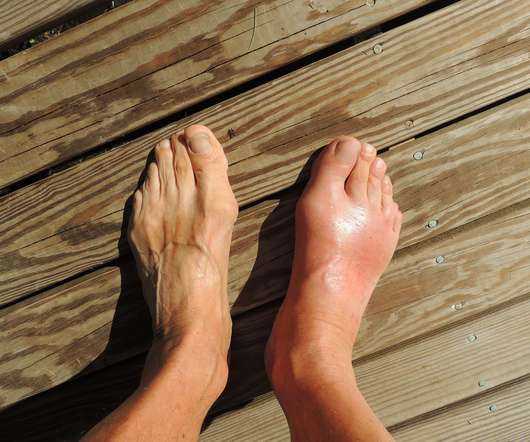
XTalks
JULY 14, 2022
Rare and rheumatic disease-focused biotech Horizon Therapeutics has received expanded approval from the US Food and Drug Administration (FDA) for its gout injection Krystexxa (pegloticase) to include its co-administration with the immunomodulator methotrexate. It is the first and only biologic approved for the condition.

pharmaphorum
NOVEMBER 24, 2020
The FDA has approved Alnylam’s gene silencing drug Oxlumo, the first treatment for primary hyperoxaluria type 1 (PH1), an ultra-rare and life-threatening genetic disorder. Alnylam is hoping that revenue will stream from its new products and payments from Novartis, which owns the rights to cholesterol lowering drug inclisiran.

XTalks
JUNE 17, 2022
Eli Lilly’s Olumiant (baricitinib) has won US Food and Drug Administration (FDA) approval as the first systemic treatment for severe alopecia areata (AA), an autoimmune disorder that leads to patchy baldness. The Olumiant approval is therefore a big win as it helps fulfill a significant unmet need in the area.

Pharmaceutical Technology
APRIL 6, 2023
The designation, under the regulator’s Innovative Licensing and Access Pathway (ILAP), will fast-track a potential route to market for AD04 by providing collaborative opportunities with UK institutes like the National Institute for Health and Care Excellence (NICE).

The Pharma Data
JANUARY 17, 2021
plans to create a global R&D achievement based on innovations of inflammation–fibrosis treatment, Triple-acting new drug for NASH (non-alcoholic steatohepatitis) treatment as well as various other innovations in metabolic disease, oncology and rare disease fields. are expected to be approved by the U.S. . U.S.

Pharmaceutical Technology
MAY 15, 2023
The US Food and Drug Administration (FDA) has approved Astellas Pharma’s Veozah (fezolinetant) for use against moderate to severe vasomotor symptoms caused by menopause on May 12 after some delays due to an extended review. The drug is currently under review in Australia, the EU and Switzerland.

XTalks
FEBRUARY 29, 2024
Two years after its approval in 2021 , Biogen has decided to pull the plug on its Alzheimer’s drug Aduhelm (aducanumab). After its accelerated approval in January 2023, Leqembi received full US Food and Drug Administration (FDA) approval in July 2023. But Biogen couldn’t convince payers of its benefits.
Pharmaceutical Technology
OCTOBER 7, 2022
Provention Bio and Sanofi US have signed a co-promotion agreement to launch the former’s lead investigational drug candidate, teplizumab for the delay in the onset of clinical type 1 diabetes (T1D). The US Food and Drug Administration (FDA) is presently reviewing teplizumab for the delay of clinical T1D in people who are at risk.

Drug Discovery World
NOVEMBER 21, 2022
The US Food and Drug Administration (FDA) has approved the Biologics License Application (BLA) for anti-CD3-directed antibody TZIELD (teplizumab-mzwv), the first immunomodulatory treatment for type 1 diabetes (T1D). .

pharmaphorum
NOVEMBER 10, 2021
Pfizer has bolstered its central nervous system (CNS) drugs portfolio with a $1.2 The drug was the first in the oral CGRP inhibitor class to get an prevention indication approved by the FDA in May, and that has helped accelerate its rollout, bringing in $336 million in US sales for Biohaven so far this year.

Drug Discovery World
JUNE 1, 2023
Antibody-drug conjugates (ADCs) are biopharmaceutical products in which a monoclonal antibody (mAB) is linked to a small molecule drug with a stable linker 1. A Nature publication confirmed that there are currently 12 FDA-approved ADCs on the market, and nine of these secured FDA approval in the past six years 2.
Pharmaceutical Technology
APRIL 25, 2023
Cidara Therapeutics announced the receipt of a $20m milestone payment from Melinta Therapeutics following the US Food and Drug Administration (FDA) approval of its antifungal treatment Rezzayo. In July 2022, Cidara gave Melinta US licensing rights for rezafungin following submission to the FDA.

XTalks
APRIL 23, 2021
GSK’s immunotherapy dostarlimab-gxly (Jemperli) has been granted accelerated approval by the US Food and Drug Administration (FDA) for the treatment of recurrent or advanced mismatch repair-deficient (dMMR) endometrial cancer.
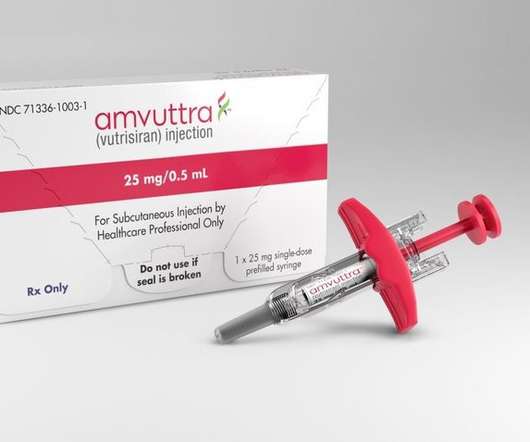
XTalks
JUNE 20, 2022
Alnylam Pharmaceuticals, a leading RNA interference (RNAi) therapeutics biopharmaceutical company, announced it received approval from the US Food and Drug Administration (FDA) for its RNAi therapeutic Amvuttra (vutrisiran) for the treatment of the polyneuropathy of hereditary transthyretin-mediated (ATTR) amyloidosis in adults.
Scienmag
MARCH 28, 2021
Octaplas™ and fibryga® receive new product labeling following FDA’s approval of BLA supplements to update therapy research; FDA expands fibryga® indication to include treatment of children under 12 years of age Credit: Octapharma USA PARAMUS, N.J. March 29, 2021) – The U.S.

pharmaphorum
DECEMBER 4, 2020
Johnson & Johnson has filed its bispecific antibody amivantamab to the FDA, hoping to muscle into the big market for drugs that are used to treat EGFR-positive non-small cell lung cancer (NSCLC). The post J&J files lung cancer bispecific amivantamab for FDA approval appeared first on.

XTalks
JANUARY 28, 2022
Kimmtrak has also become the first bispecific T cell engager to be FDA-approved for the treatment of a solid tumor. Immunocore was granted the approval for Kimmtrak several weeks ahead of the scheduled PDUFA date of February 23. Kimmtrak is part of a novel class of bispecific T cell immunotherapies being developed by Immunocore.

Drug Discovery World
NOVEMBER 25, 2022
FDA approves first disease-modifying therapy for T1D . The US Food and Drug Administration (FDA) has approved the Biologics License Application (BLA) for anti-CD3-directed antibody TZIELD (teplizumab-mzwv), the first immunomodulatory treatment for type 1 diabetes (T1D). .

Delveinsight
AUGUST 17, 2021
FDA’s Green Flag to Keytruda and Lenvima combination by Merck and Eisai for Advanced renal cell carcinoma (RCC). The FDA approved the combination of Keytruda and Lenvima produced by Merck and Eisai, as a first-line treatment of adult patients with advanced renal cell carcinoma (RCC). Jazz Pharmaceuticals announced the U.S.

XTalks
MAY 24, 2023
Eyecare giant Bausch + Lomb received its first prescription drug approval from the US Food and Drug Administration (FDA) for its dry eye disease med Miebo (perfluorohexyloctane ophthalmic solution). The drug addresses a significant unmet need for patients, said Bausch + Lomb CEO Brent Saunders in a press release.

pharmaphorum
APRIL 23, 2021
The FDA has approved GlaxoSmithKline’s Jemperli (dostarlimab) immunotherapy, a drug acquired through its $5.1 With Jemperli , a PD-1 class drug, GSK is a latecomer to the immunotherapy party with a host of other competitors already on the market in various cancers.

pharmaphorum
NOVEMBER 21, 2022
The company argues that the cost is justified as Tzield (teplizumab) is the first drug that can delay the onset of type 1 diabetes, fending off the time when they become highly reliant on insulins and at risk of the serious complications that can accompany advanced T1D. . It also has an option on global marketing rights to the drug.

Pharmaceutical Technology
MARCH 27, 2023
Iovance Bioterapeutics announced it has completed its rolling Biologics License Application (BLA) submission to the U.S. Food and Drug Administration (FDA) for Lifileucel. Marc Hurlbert, CEO of the Melanoma Research Alliance (MRA) said he hopes for a quick FDA approval.
pharmaphorum
NOVEMBER 25, 2022
The FDA has issued a complete response letter (CRL) to Spectrum Pharma for poziotinib, its pan HER2 inhibitor for a form of lung cancer, according to South Korea’s Hanmi Pharma, which originally developed the drug. Spectrum licensed worldwide rights to poziotinib – excluding Korea and China – from Hanmi in back in 2015.
XTalks
AUGUST 21, 2023
The US Food and Drug Administration (FDA) has approved Ipsen’s groundbreaking Sohonos (palovarotene) capsules for the treatment of fibrodysplasia ossificans progressive (FOP), an ultra-rare bone disease. Sohonos, the first and only approved drug, offers hope to those battling the disease.

BioPharma Reporter
APRIL 21, 2022
Last week saw the US Food and Drug Administration (FDA) approve the Biologics License Application (BLA) from Amneal Pharmaceuticals Inc for bevacizumab-maly, a biosimilar referencing Avastin, the Roche/Genentech product.

The Pharma Data
MAY 7, 2021
The Pfizer-BioNTech COVID-19 Vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA), but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 16 years of age and older.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content