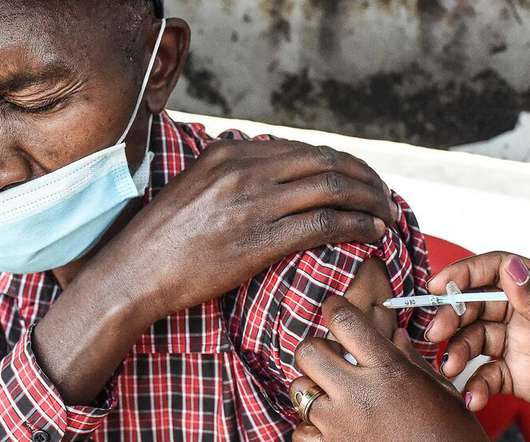
Moderna, Racing for Profits, Keeps Covid Vaccine Out of Reach of Poor
NY Times
OCTOBER 9, 2021
Some poorer countries are paying more and waiting longer for the company’s vaccine than the wealthy — if they have access at all.
NY Times
OCTOBER 9, 2021
Some poorer countries are paying more and waiting longer for the company’s vaccine than the wealthy — if they have access at all.

The Pharma Data
OCTOBER 9, 2021
If approved by the FDA, maribavir will be the first and only treatment indicated for adults in this patient population CMV is one of the most common infections experienced by transplant recipients, with an estimated incidence rate of around 16–56% in solid organ transplant (SOT) recipients and 30–70% in HSCT recipients 3,7 Regulatory submission is based on the Phase 2 and Phase 3 TAK-620-303 (SOLSTICE) trial of maribavir Maribavir is one of four Wave 1 pipeline new molecular entities that Takeda
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioTech 365
OCTOBER 9, 2021
Adverum Presents Clinical Data on ADVM-022 at the American Society of Retina Specialists 39th Annual Scientific Meeting Adverum Presents Clinical Data on ADVM-022 at the American Society of Retina Specialists 39th Annual Scientific Meeting — Company maintains focus on advancing … Continue reading →

The Pharma Data
OCTOBER 9, 2021
Gantenerumab is an investigational antibody in Phase III development for early Alzheimer’s disease (AD) Gantenerumab is the first and only anti-amyloid antibody being investigated for subcutaneous administration in late-stage trials for the treatment of AD Ongoing Phase III GRADUATE programme with gantenerumab is anticipated to deliver a comprehensive data set with expected readout in the second half of 2022.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

BioTech 365
OCTOBER 9, 2021
Biotechnology, Pharma and Biopharma News – Research – Science – Lifescience ://Biotech-Biopharma-Pharma: Chilean scientist plans to clean up mining with ‘metal eating’ bacteria.

The Pharma Data
OCTOBER 9, 2021
Application utilizes extrapolation-based strategy across existing breadth of STELARA data in patients living with this chronic inflammatory disease. The Janssen Pharmaceutical Companies of Johnson & Johnson today announced the submission of a supplemental Biologics License Application (sBLA) to the U.S. Food and Drug Administration (FDA) seeking expanded approval of STELARA® (ustekinumab) to treat pediatric patients ages 5 years and older with juvenile psoriatic arthritis (jPsA).
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

The Pharma Data
OCTOBER 9, 2021
Collaboration Leveraging Neumora’s Precision Neuroscience Platform with Amgen’s deCODE Human Genetics Capabilities to Advance Innovative Medicines for Brain Disease. Amgen and Neumora Therapeutics, a clinical-stage biopharmaceutical company pioneering precision medicines for brain diseases, announced a strategic collaboration to advance neuroscience discovery, development and commercialization.

The Pharma Data
OCTOBER 9, 2021
LATTICE-UC proof of concept study did not meet primary nor secondary endpoints. Safety profile of deucravacitinib consistent with other trials and no new safety signals reported. Bristol Myers Squibb. today announced the Phase 2 LATTICE-UC study evaluating deucravacitinib, a first-in-class, oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared to placebo in moderate to severe ulcerative colitis (UC) did not meet the primary efficacy endpoint of clinical remission at Week 12, nor secondary

The Pharma Data
OCTOBER 9, 2021
Tezepelumab has been granted Orphan Drug Designation (ODD) in the US by the Food and Drug Administration (FDA) for the treatment of eosinophilic esophagitis (EoE). Tezepelumab is being developed by AstraZeneca in collaboration with Amgen and is under Priority Review for patients with asthma in the US. The FDA grants ODD status to medicines and potential new medicines intended for the treatment, diagnosis or prevention of rare diseases or disorders that affect fewer than 200,000 people in the US.

The Pharma Data
OCTOBER 9, 2021
Novartis will present 41 abstracts from a wide-ranging multiple sclerosis (MS) portfolio, including new data on Kesimpta ® (ofatumumab) and Mayzent ® (siponimod) Novartis will be commencing Phase III pivotal trials investigating remibrutinib in RMS. Remibrutinib is a highly selective, potent oral BTK inhibitor with a potential best-in-class profile 1 Late-breaking safety data will be presented, including outcomes of COVID-19 in relapsing forms of multiple sclerosis (RMS) patients treated with

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Let's personalize your content