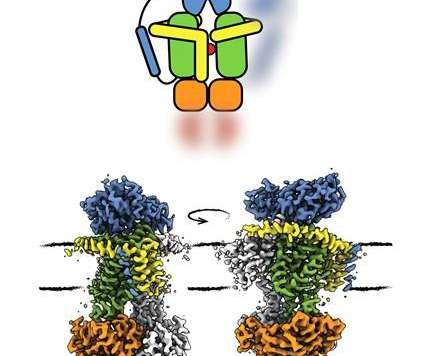
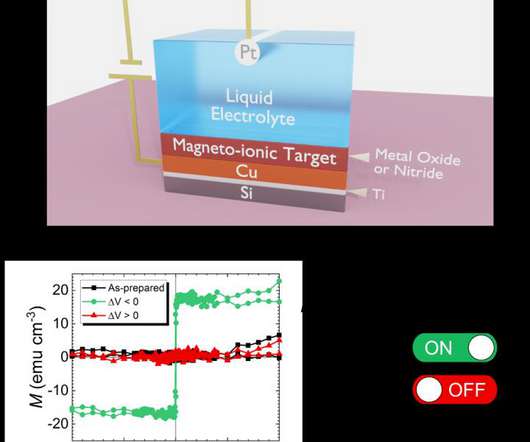
New coronavirus vaccine data back up Pfizer, BioNTech claims of strongly effective shot
Bio Pharma Dive
NOVEMBER 18, 2020
Reaching the main goal of their large trial, Pfizer and BioNTech reported 162 cases of COVID-19 among participants who received a placebo, and just 8 for those who were vaccinated.



































Let's personalize your content