A biotech wins the first FDA drug approval in a rare type of eye cancer
Bio Pharma Dive
JANUARY 26, 2022
U.K.-based Immunocore's treatment, known as Kimmtrak, is also the first T cell receptor drug to have reached market in the U.S. so far.

Bio Pharma Dive
JANUARY 26, 2022
U.K.-based Immunocore's treatment, known as Kimmtrak, is also the first T cell receptor drug to have reached market in the U.S. so far.

World of DTC Marketing
JANUARY 26, 2022
This is an exciting time to be in healthcare marketing. We’re witnessing the most significant transformation in healthcare marketing since DTC was approved in the ’90s. We’re moving from an era of “promotional marketing” to an age of “data is key” and “helping patients become consumers of healthcare.” HCP’s are moving quickly into digital.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Bio Pharma Dive
JANUARY 26, 2022
The study will test a version of the biotech's COVID-19 shot that's tailored to the infectious variant. New data, meanwhile, help affirm the benefit of a third dose of Moderna's current vaccine.

pharmaphorum
JANUARY 26, 2022
Immunocore has secured a piece of biotech industry history, becoming the first company to get an FDA approval for a cancer therapeutic based on T cell receptor (TCR) technology. The US regulator cleared the company’s lead TCR Kimmtrak (tebentafusp; formerly IMCgp100 ) – a bispecific T cell engager (BiTE) that consists of a soluble TCR targeting gp100 expressed on cancer cells fused to a protein that binds CD3 receptors on lymphocytes – as a treatment for unresectable or metastatic uveal me

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Bio Pharma Dive
JANUARY 26, 2022
U.S. regulators halted several studies of a drug Gilead paid almost $5 billion to acquire in 2020 after researchers observed an "apparent imbalance" of serious adverse reactions.
Eye on FDA
JANUARY 26, 2022
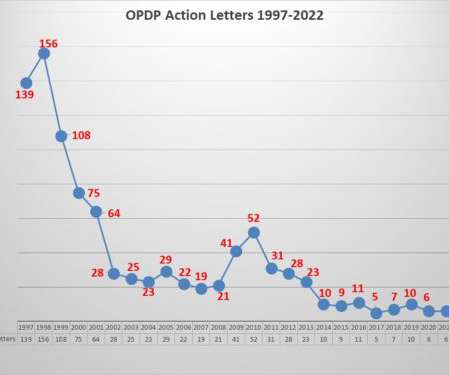
FDA’s Office of Prescription Drug Promotion (OPDP) issued the first regulatory action letter for 2022. This one has some notable characteristics. As has been frequently noted, enforcement actions by OPDP have diminished considerably over the years. Another characteristic of recent enforcement actions over the past several years is that smaller, less well-known companies have mostly been the recipients of letters from OPDP.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

pharmaphorum
JANUARY 26, 2022
The final numbers are in, and now it’s official – digital health companies raked in a massive $57.2 billion in funding last year, beating prior records with a 79% increase over 2020. The data from CB Insights’ annual State of Digital Health report notes that the record investments were apparent across all geographies and were “fuelled by the growing need to provide digital solutions and delivery models to patients during the pandemic.” Highlights of the year included an a

Bio Pharma Dive
JANUARY 26, 2022
The decision is a further sign of regulators' scrutiny of accelerated approvals for cancer therapies, an initiative that's led to several market withdrawals.
BioPharma Reporter
JANUARY 26, 2022
Japanâs ministry for health has approved Abecma as a CAR T-cell therapy for adults with relapsed or refractory multiple myeloma who received at least three prior therapies.

pharmaphorum
JANUARY 26, 2022
We have seen a boom in biotech initial public offerings over the last two years, but many of Europe’s offerings have migrated to the United States. So, what can we do to keep home-grown science on European soil? From the Pfizer/BioNTech and Oxford/AstraZeneca vaccines to GSK’s lifesaving antibody treatments, European innovation has played a crucial role in the global response to COVID-19.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

BioPharma Reporter
JANUARY 26, 2022
Contract packaging and clinical supply services company Sharp has completed the construction of new purpose-built production suites to facilitate the packaging, storage and distribution of gene therapies at its facility in Heerenveen, The Netherlands.

XTalks
JANUARY 26, 2022
A field application scientist is a valuable member of any life science company due to their direct interaction with customers. Field application scientists help laboratory groups successfully install and apply the newest scientific supplies, equipment, instruments and software. An application scientist typically works for life science companies that manufacture laboratory tools or provide a service for laboratory use.

BioPharma Reporter
JANUARY 26, 2022
Last year was the highest year on record for investments into UK biotech and life science companies: with Â4.5bn ($6bn) raised in public and private financings, representing 60% more than in 2020.

XTalks
JANUARY 26, 2022
In this episode, Ayesha discussed Gilead Science’s lawsuit against a network of distributors selling counterfeits of the company’s best-selling HIV drugs Biktarvy and Descovy. The drugs were being sold in pharmacies across several states. Gilead said it had been working with the US marshals and local law enforcement and had so far seized more than 85,000 counterfeit drug bottles.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

BioPharma Reporter
JANUARY 26, 2022
Netherlands-based gene therapy company, VectorY Therapeutics, and Wageningen University have signed a strategic collaboration agreement for the development of novel baculovirus-based AAV production technologies.
NY Times
JANUARY 26, 2022
Myocarditis, or inflammation of the heart muscle, occurred in 1 of 12,361 boys between 12 and 15 years old within a week of receiving a second dose of the Pfizer-BioNTech vaccine, researchers found.
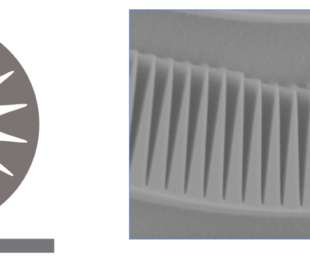
Scienmag
JANUARY 26, 2022
AMHERST, Mass. — Researchers including a postdoc at the University of Massachusetts Amherst have created a gear-shaped photonic crystal microring that increases the strength of light-matter interactions without sacrificing optical quality. The result is an on-chip microresonator with an optical quality factor 50 times better than the previous record in slow light devices that could […].

Pharma Times
JANUARY 26, 2022
COVID-19 vaccine luminaries Pfizer and BioNTech study will examine data from the current booster and consider need for specific Omicron jab

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Scienmag
JANUARY 26, 2022
Researchers have discovered what appears to be the earliest known account of a rare weather phenomenon called ball lightning in England. Credit: The Master and Fellows of Trinity College, Cambridge. Reference: Cambridge, Trinity College, MS R.4.11, p.324. Researchers have discovered what appears to be the earliest known account of a rare weather phenomenon called ball […].
Outsourcing Pharma
JANUARY 26, 2022
A leader from Elligo Health Research (a SCOPE 2022 exhibitor ) discusses flaws in traditional recruitment approaches and offers up some fresh new ideas.

Pharma Times
JANUARY 26, 2022
Ipsen and Domainex collaboration will provide an exclusive licence to develop innovative inhibitors and pioneering cancer treatments
Scienmag
JANUARY 26, 2022
WHAT: In adults who had previously received a full regimen of any of three COVID-19 vaccines granted Emergency Use Authorization (EUA) or approved by the Food and Drug Administration (FDA), an additional booster dose of any of these vaccines was safe and prompted an immune response, according to preliminary clinical trial results reported in The […].

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

BioTech 365
JANUARY 26, 2022
Epizyme Announces Proposed Public Offering of Common Stock Epizyme Announces Proposed Public Offering of Common Stock CAMBRIDGE, Mass.–(BUSINESS WIRE)–Epizyme, Inc.
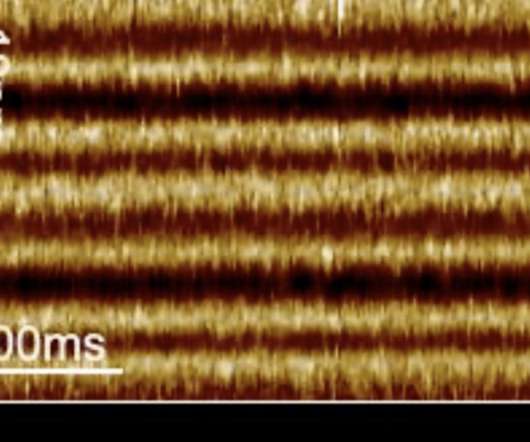
Scienmag
JANUARY 26, 2022
Using an innovative new imaging technique, researchers at Weill Cornell Medicine have revealed the inner workings of a family of light-sensing molecules in unprecedented detail and speed. The work could inform new strategies in the burgeoning field of optogenetics, which uses light pulses to alter the activity of individual neurons and other cells. Credit: Image […].

NY Times
JANUARY 26, 2022
It never quite fit in your wallet, so it’s no surprise you may have misplaced it by now.

Scienmag
JANUARY 26, 2022
A new UBC study confirms that when men transition out of relationships, they are at increased risk of mental illness, including anxiety, depression and suicide. Credit: University of British Columbia A new UBC study confirms that when men transition out of relationships, they are at increased risk of mental illness, including anxiety, depression and suicide. […].

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Outsourcing Pharma
JANUARY 26, 2022
The upcoming industry event offers attendees (in-person and virtual) a range of opportunities to learn, connect, and innovate, according to an organizer.
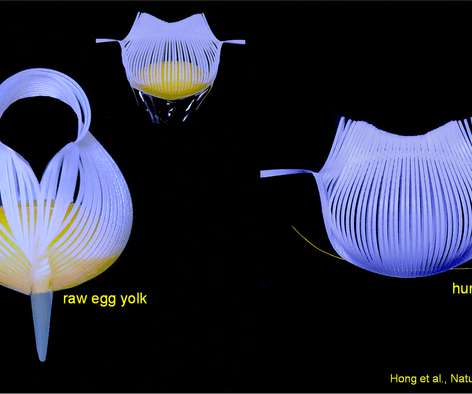
Scienmag
JANUARY 26, 2022
Engineering researchers from North Carolina State University have demonstrated a new type of flexible, robotic grippers that are able to lift delicate egg yolks without breaking them, and that are precise enough to lift a human hair. The work has applications for both soft robotics and biomedical technologies. Credit: Jie Yin, North Carolina State University […].
Drug Patent Watch
JANUARY 26, 2022
Annual Drug Patent Expirations for BIJUVA Bijuva is a drug marketed by Therapeuticsmd Inc and is included in one NDA. It is available from one supplier. There are nineteen patents…. The post New patent for Therapeuticsmd Inc drug BIJUVA appeared first on DrugPatentWatch - Make Better Decisions.

Scienmag
JANUARY 26, 2022
There is no crystal ball to tell ecologists how forests of the future will respond to the changing climate, but a University of Arizona-led team of researchers may have created the next best thing. Credit: N/A There is no crystal ball to tell ecologists how forests of the future will respond to the changing climate, […].

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content