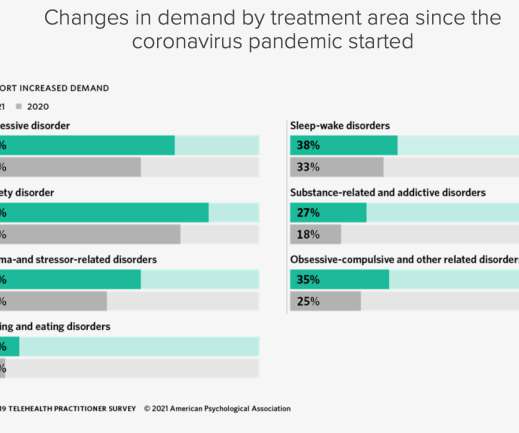
The need for mental healthcare is great
World of DTC Marketing
MARCH 27, 2022
Mental illnesses are common in the United States. Nearly one in five U.S. adults live with a mental illness (52.9 million in 2020). Mental illnesses include many different conditions that vary in severity, ranging from mild to moderate to severe. Suicidal ideation continues to increase among adults in the U.S. 4.58% of adults report having serious thoughts of suicide, an increase of 664,000 people from last year’s dataset.

















Let's personalize your content