New Year, New Price Hikes on Hundreds of Prescription Drugs
BioSpace
JANUARY 3, 2021
As the sun rose on a new year, approximately 70 companies raised the price of hundreds of prescription drugs by an average of 3.3%.

BioSpace
JANUARY 3, 2021
As the sun rose on a new year, approximately 70 companies raised the price of hundreds of prescription drugs by an average of 3.3%.

JAMA Internal Medicine
JANUARY 3, 2021
The rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has imposed multilevel challenges on societies everywhere, from abrupt changes in our day-to-day lives to overwhelming new pressures on health systems worldwide. Although there are many devastating effects of coronavirus disease 2019 (COVID-19), few compare with the pandemic’s terrible burden on nursing home residents, their families, and their health care clinicians.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioSpace
JANUARY 3, 2021
As Operation Warp Speed struggles to ramp up vaccinations, one of the approaches under consideration is cutting the doses of the Moderna vaccine.

JAMA Internal Medicine
JANUARY 3, 2021
This cohort study examines risk factors for 30-day all-cause mortality among US nursing home residents with COVID-19.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
BioSpace
JANUARY 3, 2021
This news, published in the American Journal of Human Genetics, enables researchers to develop early screening methods before the most serious symptoms arise, and thus intervene.

The Pharma Data
JANUARY 3, 2021
SOUTH SAN FRANCISCO, Calif., Jan. 04, 2021 (GLOBE NEWSWIRE) — Calithera Biosciences, Inc. (Nasdaq: CALA), a clinical-stage biotechnology company focused on discovering and developing novel small-molecule drugs for the treatment of cancer and other life-threatening diseases, today announced topline results from the CANTATA clinical study of the company’s glutaminase inhibitor telaglenastat in patients with advanced or metastatic renal cell carcinoma (RCC).
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

The Pharma Data
JANUARY 3, 2021
nitpicker/Shutterstock. The $9 Celgene Contingent Value Rights (CVR) payout is dead. The clock struck midnight on New Year’s Eve, ushering in a new year, and the U.S. Food and Drug Administration (FDA) did not approve the cancer drug liso-cel (lisocabtagene maraleucel) by the required Dec. 31 deadline. The Dec. 31 deadline was one of three required milestones for the $9 CVR.

BioSpace
JANUARY 3, 2021
Pfizer and OPKO Health are one step closer to receiving approval for their jointly developed pediatric growth hormone deficiency drug, somatrogon, after the U.S. FDA recently accepted the companies’ regulatory submission for the therapy.

The Pharma Data
JANUARY 3, 2021
U.S. Food and Drug Administration cleared Investigational New Drug Application. United Kingdom Medicines and Healthcare Products Regulatory Agency authorized Clinical Trial Application. Program addressing rare mineralization disorders expected to enroll first subject in H1’21 and provide preliminary safety and biomarker data in H2’21. BOSTON, Jan. 04, 2021 (GLOBE NEWSWIRE) — Inozyme Pharma, Inc.

BioSpace
JANUARY 3, 2021
Here’s a look at the top 10 novel drug approvals of 2020, loosely based on projected earnings in the upcoming years.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

The Pharma Data
JANUARY 3, 2021
SOUTH SAN FRANCISCO, Calif. and TAIPEI, Taiwan, Jan. 04, 2021 (GLOBE NEWSWIRE) — TLC (Nasdaq: TLC, TWO: 4152), a clinical-stage specialty pharmaceutical company developing novel nanomedicines to target areas of unmet medical need, announced today that its subsidiary, InspirMed Inc., has completed a round of financing, raising US$15 million in cash from strategic investors in exchange for subsidiary equity.

BioSpace
JANUARY 3, 2021
Without an approval for liso-cel, Bristol Myers Squibb said the CVRs have now been terminated, are no longer eligible for payment and will no longer be allowed to trade on the New York Stock Exchange.

The Pharma Data
JANUARY 3, 2021
WATERTOWN, Mass., Jan. 03, 2021 (GLOBE NEWSWIRE) — EyePoint Pharmaceuticals, Inc. (NASDAQ: EYPT), a pharmaceutical company committed to developing and commercializing innovative ophthalmic products, today announced that Ocumension Therapeutics, a China-based ophthalmic pharmaceutical company traded on the Stock Exchange of Hong Kong (1477.HK), has made a $15.7 million equity investment in EyePoint.

BioSpace
JANUARY 3, 2021
Dr. Lynn Seely bows out as Myovant pivots to commercialize new drug.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

The Pharma Data
JANUARY 3, 2021
Drug Industry Daily (DID) the premier online resource for savvy pharmaceutical professionals whose jobs depend on accurate knowledge about the daily activities of Congress, the FDA, other key regulators … and what their competitors are up to. Don’t waste your time on unending, unproductive online searches for news stories, reports and opinion; let DID’s experienced in-house editorial team do the work for you.
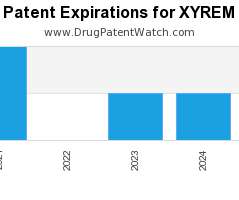
Drug Patent Watch
JANUARY 3, 2021
Annual Drug Patent Expirations for XYREM Xyrem is a drug marketed by Jazz Pharms and is included in one NDA. It is available from one supplier. There are fourteen patents…. The post New patent expiration for Jazz Pharms drug XYREM appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
JANUARY 3, 2021
Regeneron has released positive initial results from an ongoing phase 1/2/3 study evaluating its antibody cocktail, casirivimab and imdevimab, in hospitalized COVID-19 patients who require low-flow oxygen. Source link.

Drug Patent Watch
JANUARY 3, 2021
Annual Drug Patent Expirations for XYREM Xyrem is a drug marketed by Jazz Pharms and is included in one NDA. It is available from one supplier. There are fourteen patents…. The post New patent expiration for Jazz Pharms drug XYREM appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

The Pharma Data
JANUARY 3, 2021
Anecdotal feedback from Foralumab-treated patients was positive and suggests that the treatment was well-tolerated. The scientific approaches underlying this clinical study could potentially be effective against SARs, MERS, and all variants of coronaviruses. This trial is the first to evaluate nasally administered Foralumab to improve the immune system’s fight against coronaviruses. NEW YORK and LONDON, Jan. 04, 2021 (GLOBE NEWSWIRE) — Tiziana Life Sciences plc (Nas

Drug Patent Watch
JANUARY 3, 2021
CLEVIPREX (clevidipine) Chiesi Patent: 5,856,346 Expiration: Jan 5, 2021 See More … For more information on how DrugPatentWatch can help with your pharmaceutical business intelligence needs, contact admin@DrugPatentWatch.com or visit…. The post Drug Patent Expirations for the Week of January 3, 2021 appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
JANUARY 3, 2021
HELSINKI , Jan. 4, 2021 /PRNewswire/ — Nanoform Finland Plc (“Nanoform”), an innovative nanoparticle medicine enabling company, today announced a new near-term business target. In 2021 Nanoform targets “at least 12 new non-GMP 1 (pre-clinical) customer projects and at least one new GMP (clinical) customer project” The company reported 2 non-GMP customer projects in 2019 and 7 non-GMP customer projects in Q1-Q3/2020.

Drug Patent Watch
JANUARY 3, 2021
CLEVIPREX (clevidipine) Chiesi Patent: 5,856,346 Expiration: Jan 5, 2021 See More … For more information on how DrugPatentWatch can help with your pharmaceutical business intelligence needs, contact admin@DrugPatentWatch.com or visit…. The post Drug Patent Expirations for the Week of January 3, 2021 appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

The Pharma Data
JANUARY 3, 2021
SHENZHEN, China , Jan. 4, 2021 /PRNewswire/ — CIMC Enric Holdings Limited (“ CIMC Enric ” or “ the Company “, SEHK stock code: 3899.HK), announced that its subsidiary Zhangjiagang CIMC Sanctum Cryogenic Equipment Co., Ltd (“ CIMC Sanctum “) recently started R&D of liquid nitrogen biological containers for long-term cryogenic storage of vaccines, stem cells, plasma, semen, embryos and various tissues and organs for the needs of the biomedical industry

BioTech 365
JANUARY 3, 2021
EyePoint Pharmaceuticals Announces $15.7 Million Equity Investment by Asia Partner Ocumension Therapeutics EyePoint Pharmaceuticals Announces $15.7 Million Equity Investment by Asia Partner Ocumension Therapeutics WATERTOWN, Mass., Jan. 03, 2021 (GLOBE NEWSWIRE) — EyePoint Pharmaceuticals, Inc.

The Pharma Data
JANUARY 3, 2021
Zeldis formerly served as Celgene CMO and CEO of Celgene Global Health. Weber is a renowned melanoma specialist and leading immunotherapy translational and clinical scientist. Both will help NexImmune advance current early-stage clinical trials and will guide Company’s translational efforts to develop new immunotherapy products. GAITHERSBURG, Md., Jan. 04, 2021 (GLOBE NEWSWIRE) — NexImmune , a clinical-stage biotechnology company developing a novel approach to immun

BioTech 365
JANUARY 3, 2021
DentaFend Reviews 2021 – Ingredients Really Work or Fake Results? Independent Review by FitLivings DentaFend Reviews 2021 – Ingredients Really Work or Fake Results? Independent Review by FitLivings DentaFend supplement reviews.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

The Pharma Data
JANUARY 3, 2021
Jan. 4, 2021 11:00 UTC. PHOENIX–( BUSINESS WIRE )– Nextmune , the leader in allergy, dermatology, otology and specialized nutrition for pets, is pleased to announce that the company’s businesses – Dr. Baddaky (Scandinavia), Vetruus (UK), Artuvet (Germany, Benelux), Alergovet (Iberia) and Spectrum Vet (US) – will begin operating under a new name and will be known as Nextmune starting 1 January, 2021.
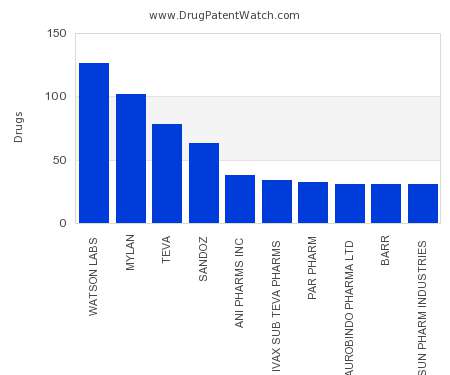
Drug Patent Watch
JANUARY 3, 2021
This chart shows the pharmaceutical companies with the most capsule dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most capsule dosed drugs…. The post Which pharmaceutical companies have the most capsule dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
JANUARY 3, 2021
PHILADELPHIA, Jan. 04, 2021 (GLOBE NEWSWIRE) — Passage Bio , Inc. (Nasdaq: PASG), a genetic medicines company focused on developing transformative therapies for rare monogenic central nervous system (CNS) disorders, today announced that U.S. Food and Drug Administration (FDA) has cleared an investigational new drug (IND) application for the company’s lead product candidate, PBGM01, an adeno-associated virus (AAV)-delivery gene therapy that is being studied for the treatment of infant

Drug Patent Watch
JANUARY 3, 2021
This chart shows the pharmaceutical companies with the most capsule dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most capsule dosed drugs…. The post Which pharmaceutical companies have the most capsule dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content