Pharma’s big lie is about greed
World of DTC Marketing
DECEMBER 17, 2021
The public has been told that pharma needs money to develop new drugs, but unfortunately, that’s a huge lie. As biopharmadive recently, “some of the biggest pharmaceutical companies, sitting on large and growing sums of cash, are funneling those funds into major share buyback programs and acquisitions of smaller biotech companies. On Thursday, Swiss drug-making giant Novartis announced plans to buy back up to $15 billion worth of its shares by end of 2023, a program it will fund wi

















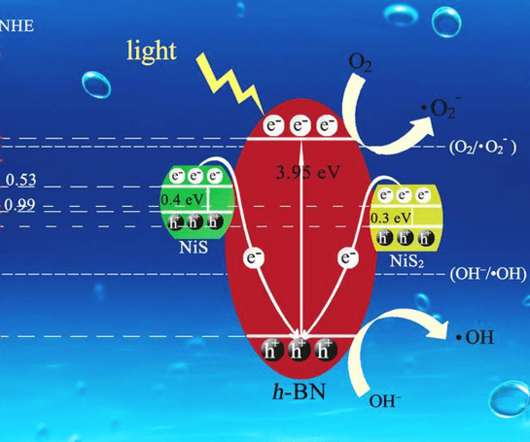



















Let's personalize your content