Argentina, Mexico line up to produce AstraZeneca, Oxford coronavirus vaccine
Bio Pharma Dive
AUGUST 13, 2020
The manufacturing deal could help supply Latin America with Oxford's shot, which is one of the most advanced in clinical testing.
Bio Pharma Dive
AUGUST 13, 2020
The manufacturing deal could help supply Latin America with Oxford's shot, which is one of the most advanced in clinical testing.

World of DTC Marketing
AUGUST 13, 2020
IN BRIEF: Pharma companies have been posting jobs on LinkedIn almost every day. While most offices remain closed pharma is preparing for the launch of new products and has to have the infrastructure to ensure a successful launch. I keep in touch with all my clients via email newsletters and ask them to “stay in touch” It seems that this year, I’ve been seeing more people leave for other jobs within the industry or clients asking me to suggest people for open positions.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Bio Pharma Dive
AUGUST 13, 2020
The biotech, which has quickly advanced its coronavirus shot to late-stage trials, raised nearly $750 million through the listing.

Camargo
AUGUST 13, 2020
Each month, Camargo’s “In the News” series will highlight important changes and advancements in the. The post In the News: July Regulatory and Development Updates appeared first on Camargo.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Bio Pharma Dive
AUGUST 13, 2020
Thermo Fisher had raised its bid for Qiagen to about $12.5 billion, but less than half of the company's shares were tendered by the offer's expiration Monday.
Pharma Mirror
AUGUST 13, 2020
Tadalafil is a prescription drug well-known to men over the age of 40 who usually have to deal with episodic or permanent erectile dysfunction. Yet there’s more to this substance than its typical use in managing impotence by improving the blood flow to the genitals. The substance is used in many other treatments and has. The post What Exactly Is Tadalafil?
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharma Times
AUGUST 13, 2020
If approved drug would offer a more flexible option for patients
BioSpace
AUGUST 13, 2020
Phase III clinical trials for Russia’s Sputnik V vaccine for COVID-19 began Wednesday, one day after the Russia Direct Investment Fund (RDIF) launched a new website to share the details of the vaccine with the public and scientists around the world.

XTalks
AUGUST 13, 2020
Up to 750,000 COVID-19 test kits manufactured by Randox Laboratories have been recalled in the UK. Randox Laboratories, a Northern Ireland-based medical technology company, has been informed to recall the tests as a precautionary measure. The kits have been recalled over concerns that they do not meet the required safety standards and the company has been instructed by the British government to stop distributing and using the COVID-19 test kits as of July 15.
BioSpace
AUGUST 13, 2020
Novavax entered into an agreement to provide the United Kingdom with 60 million doses of the vaccine should it be approved, as well as a late-stage efficacy study in that country.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Scienmag
AUGUST 13, 2020
Credit: UTHSC Memphis, Tenn. (August 13, 2020) – Ansley Grimes Stanfill, PhD, RN, associate dean of Research for the UTHSC College of Nursing, has been selected for the American Academy of Nursing’s 2020 Class of Fellows. Dr. Stanfill is also an alumna of UTHSC, earning her PhD in Nursing Science at the university in 2014. […].

BioSpace
AUGUST 13, 2020
Niclosamide is an oral anthelminthic drug. It was first approved by the U.S. Food and Drug Administration (FDA) in 1982 and is included in the World Health Organization (WHO)’s list of essential medicines.

pharmaphorum
AUGUST 13, 2020
How the COVID-19 pandemic is forging pharmaceutical brands into better shape. Day by day it becomes clearer that some brands are coming through the pandemic in better shape than ever. These are the brands that have clearly demonstrated emotional intelligence, that have shown emotional empathy to their target audience. They understand the current, heightened emotional state of their customers and respond to it in ways that show they give a damn.
BioSpace
AUGUST 13, 2020
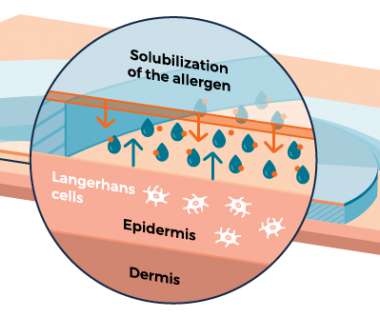
DBV Technologies aims to fill the peanut allergy treatment gap, especially for highly allergic children, with their low dose epicutaneous immunotherapy (EPIT) patch Viaskin™ Peanut.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Scienmag
AUGUST 13, 2020
Credit: Jason Kaye Research Group, Penn State To judge the overall effectiveness of cover crops and choose those offering the most ecosystem services, agricultural scientists must consider the plants’ roots as well as above-ground biomass, according to Penn State researchers who tested the characteristics of cover crop roots in three monocultures and one mixture. “Almost […].
BioSpace
AUGUST 13, 2020
The results from a three-month study in more than 35,000 patients were published yesterday on a preprint server, which means they have not yet been peer-reviewed.
Scienmag
AUGUST 13, 2020
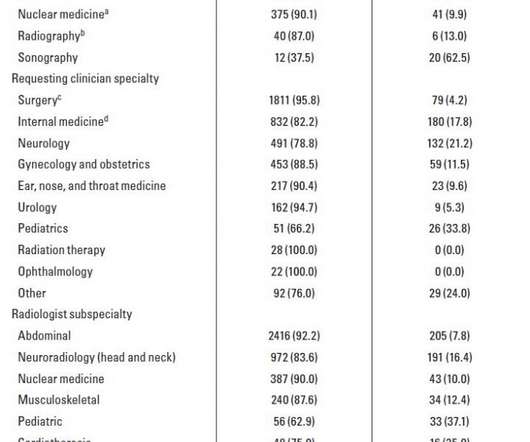
AJR finds clinicians not reading a considerable proportion (11.4%) of second-opinion radiology reports, especially sonography, pediatrics, interventional radiology Credit: American Roentgen Ray Society (ARRS), American Journal of Roentgenology (AJR) Leesburg, VA, August 13, 2020–According to ARRS’ American Journal of Roentgenology (AJR), clinicians do not read a considerable proportion of second-opinion radiology reports–“a situation that can […].
XTalks
AUGUST 13, 2020
As the rush for a COVID-19 vaccine continues, healthcare services company Kaiser Permanente has joined in on the clinical testing of Pfizer Inc. and BioNTech’s lead vaccine candidate in a Phase III trial being conducted at sites in California and Oregon. The company announced that it commenced the Phase III clinical trial to evaluate the investigational COVID-19 vaccine this week.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Scienmag
AUGUST 13, 2020
New solar cells for space Credit: Wei Chen / TUM Perovskite and organic solar cells are promising options for future generations of solar cells. Over recent years, their efficiency has rapidly caught up with that of conventional silicon-based cells. “The best perovskite solar cells currently achieve efficiency levels of 25 percent,” says Peter Mueller-Buschbaum, Professor […].
BioSpace
AUGUST 13, 2020
Every week there are numerous scientific studies published. Here’s a look at some of the more interesting ones.

Scienmag
AUGUST 13, 2020
Simple innovation expected to open multiple new avenues for quantum science Credit: University of Chicago If we can harness it, quantum technology promises fantastic new possibilities. But first, scientists need to coax quantum systems to stay yoked for longer than a few millionths of a second. A team of scientists at the University of Chicago’s […].

BioSpace
AUGUST 13, 2020
Please check out the biopharma industry coronavirus (COVID-19) stories that are trending for August 14, 2020.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
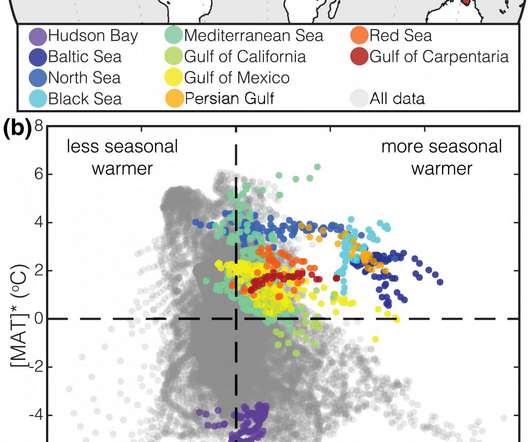
Scienmag
AUGUST 13, 2020
Credit: Syracuse University A key component when forecasting what the Earth’s climate might look like in the future is the ability to draw on accurate temperature records of the past. By reconstructing past latitudinal temperature gradients (the difference in average temperature between the equator and the poles) researchers can predict where, for example, the jet […].

BioPharma Reporter
AUGUST 13, 2020
US government inks deal to buy 100 million COVID-19 vaccine doses from Moderna for $1.5 billion, with more to follow.
Pharma Times
AUGUST 13, 2020
UK biopharma company signs licensing agreement for emactuzumab

Scienmag
AUGUST 13, 2020
Xin Ning, assistant professor of aerospace engineering, was awarded a National Science Foundation grant to translate foldable space tools into medical equipment on Earth Xin Ning, an assistant professor of aerospace engineering, specializes in developing materials for use in space. He has now received a National Science Foundation (NSF) grant to apply his research a […].

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharma Times
AUGUST 13, 2020
Public trial will launch today in Isle of Wight and other locations

Scienmag
AUGUST 13, 2020
Credit: Teiksma Buseva Employers in Sweden more often reject job applications from transgender people – especially in male-dominated occupations. Moreover, transgender people face discrimination from two different grounds for discrimination. This is according to a study from Linköping University that was recently published in the journal Labour Economics.

XTalks
AUGUST 13, 2020
Recruiters know that finding top talent in the life sciences is challenging. With hiring managers currently navigating virtual interviews and remote onboarding, it’s more important than ever to find the best-qualified applicants. Making use of highly-targeted job posting sites to narrow the candidate pool can make the hiring process easier. To meet this growing need, Xtalks has launched Xtalks Job Search , a fully customizable tool to help bring life science employers and job seekers together.

BioSpace
AUGUST 13, 2020
You’ve had your job for a while now. You’ve gotten multiple great reviews. You’ve consistently taken on additional projects and excelled at them. But you have yet to receive a promotion and you’re left wondering “Why?”.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content