Pfizer taps McKinsey executive to lead dealmaking efforts
Bio Pharma Dive
AUGUST 26, 2021
Aamir Malik, a managing partner at the consulting firm, will replace longtime Pfizer executive John Young as chief business innovation officer next week.

Bio Pharma Dive
AUGUST 26, 2021
Aamir Malik, a managing partner at the consulting firm, will replace longtime Pfizer executive John Young as chief business innovation officer next week.

World of DTC Marketing
AUGUST 26, 2021
SUMMARY : Via Marci Hamilton is a professor at the University of Pennsylvania “the Constitution is not a suicide pact guaranteeing a right to harm others. The government has latitude to protect citizens from deadly conditions, especially when the science supporting vaccination is so clear” Prof Hamilton goes on to say, “One frequently heard pushback against vaccine mandates is that there is a “constitutional right” to choose whether to be vaccinated or not for adult
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
AUGUST 26, 2021
A federal appeals court overturned an earlier verdict that had ruled Gilead's Kite Pharma unit infringed on a patent held by Bristol Myers Squibb.

pharmaphorum
AUGUST 26, 2021
A merger between two digital mental health companies – Headspace and Ginger – will create a $3 billion giant in a category that has been growing at breakneck speed as a result of the COVID-19 pandemic. The deal brings together Headspace’s meditation and mindfulness app – used by millions of people around the world – and Ginger’s platform which provides therapy and psychiatric support as an on-demand, video-based service.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Bio Pharma Dive
AUGUST 26, 2021
With positive results from a Phase 3 trial in hand, the British pharma intends to soon seek regulatory approval for the Wilson disease treatment.

BioSpace
AUGUST 26, 2021
The combined company out of the Humacyte SPAC will be called Humacyte and is expected to trade shares of common stock and warrants on the Nasdaq Global Select Market.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

BioPharma Reporter
AUGUST 26, 2021
A new partnership promises improved biomanufacturing processes for gene therapies; the goal is to support the sector in scaling up through more efficient, cost-effective processes.

BioSpace
AUGUST 26, 2021
Approximately 14,000 American women are expected to be diagnosed with invasive cervical cancer in 2021, and more than 4,000 are expected to succumb to the disease.

XTalks
AUGUST 26, 2021
How has the COVID-19 pandemic changed the way brands and retailers hand out food samples? In this episode of the Xtalks Food Podcast, Sydney discusses how food sampling has changed in retail settings and at home, with many companies shifting to e-commerce or online grocery orders as a means to send out samples. The team discusses whether in-store samples will return to their pre-pandemic state and why sample booths are crucial for small and emerging businesses.
BioSpace
AUGUST 26, 2021
?Scientists from the U.K. conducted a massive study on the link between vaccinations and dropping platelet counts and found that the problem lies more on the virus itself than on the medications.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

XTalks
AUGUST 26, 2021
In this episode, Sarah presents three ways that Alzheimer’s trials will change now that Biogen’s Aduhelm (aducanumab) has been approved by the FDA. The team discusses whether placebo-controlled trials are still ethical and how ongoing dementia studies can manage patient attrition. Can trials pivot to allow patients to aducanumab as a background therapy?

BioSpace
AUGUST 26, 2021
The bill may lead to a 15% to 25% reduction in expected returns for drugs in the top quintile of expected returns. Meaning a decrease in new medications entering the market in the following years.

Delveinsight
AUGUST 26, 2021
Shape Therapeutics inks gene therapy deal with Roche worth up to USD 3 Billion. Seattle biotech firm Shape Therapeutics has signed a deal potentially exceeding USD 3 billion with pharma giant Roche to bolster the development of gene therapies for Alzheimer’s and Parkinson’s disease. Shape’s RNA editing technologies can modify the RNA sequence, which makes the body’s protein building blocks.

FDA Law Blog
AUGUST 26, 2021
By JP Ellison — It is a well-accepted fact that even well run ethical and compliant organizations can have serious problems. The Sentencing Guidelines, the DOJ Manual, and the HHHS OIG–among others–all recognize that reality. In its Compliance Guidance for Pharmaceutical Manufacturers , the HHS OIG notes: The OIG recognizes that the implementation of a compliance program may not entirely eliminate improper conduct from the operations of a pharmaceutical manufacturer.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharma Times
AUGUST 26, 2021
Early stage research found 5-azacytidine may be used to treat 'devastating' disease

BioPharma Reporter
AUGUST 26, 2021
cold chain logistics provider, Cryoport, has established a multi-year strategic partnership with Japanâs Mitsubishi Logistics Corporation (MLC).

BioSpace
AUGUST 26, 2021
At the six-month mark, 68% of participants reported at least one persistent symptom, which fell to 49% after one year.

BioPharma Reporter
AUGUST 26, 2021
Brazilâs Eurofarma LaboratÃrios SA is to manufacture Pfizer and BioNTech's COVID-19 vaccine, Comirnaty, for distribution within Latin America.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharma Times
AUGUST 26, 2021
Up to 8,000 people per day can access the tests
BioSpace
AUGUST 26, 2021
Every week there are numerous scientific studies published. Here’s a look at some of the more interesting ones.

Pharma Times
AUGUST 26, 2021
Phase III trial evaluated use in infants 42-90 days old

BioSpace
AUGUST 26, 2021
Gilead won the most recent battle when a U.S. appeals court threw out a $1.2 billion ruling against the company.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

Pharma Times
AUGUST 26, 2021
Korsuva injection will treat CKD patients undergoing haemodialysis

BioSpace
AUGUST 26, 2021
An alleged plan by Johnson & Johnson to spin off a company solely responsible for its talc-based products in order to mitigate lawsuit damages is still on the table following a ruling by a federal judge.
pharmaphorum
AUGUST 26, 2021
Three years ago, Johnson & Johnson set up a wide-ranging initiative with Boston University to try to improve lung cancer survival rates through prevention and early intervention. Now, its calling on artificial intelligence specialist Optellum to help with that effort. UK-based Optellum has developed an AI-powered clinical decision support – called Virtual Nodule Clinic – that it says acts as a “digital biomarker”, looking for signs of cancer in computed tomography (CT) scans.

Outsourcing Pharma
AUGUST 26, 2021
A study conducted by trial tech firms Curedatis and Climedo Health reveals what trial professionals view as challenges and opportunities in digitalization.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

BioSpace
AUGUST 26, 2021
Biopharma companies and life sciences organizations strengthen their leadership teams and boards with these Movers & Shakers.

BioPharma Reporter
AUGUST 26, 2021
The International Vaccine Institute (IVI) and Bharat Biotech have started a Phase 2/3 clinical trial for a chikungunya vaccine candidate in Costa Rica.
Drug Patent Watch
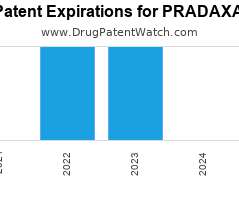
AUGUST 26, 2021
Annual Drug Patent Expirations for PRADAXA Pradaxa is a drug marketed by Boehringer Ingelheim and is included in two NDAs. It is available from three suppliers. There are five patents…. The post New patent for Boehringer Ingelheim drug PRADAXA appeared first on DrugPatentWatch - Make Better Decisions.

BioPharma Reporter
AUGUST 26, 2021
The European Medicines Agencyâs human medicines committee (EMA CHMP) has this week approved additional manufacturing capacity for Pfizer/BioNTech and Moderna COVID-19 vaccines.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content