Once again it was a quiet year with respect to the FDA’s Office of Prescription Drug Programs (OPDP). As noted in the past, there are two primary means for understanding the agency’s latest thinking with respect to promotional communications from pharmaceutical companies – the content and pattern of enforcement and the issuance of guidance documents to shed light on the parameters that exist. In that respect, 2021 was not a banner year.
The Numbers: Looking first at the quantity of enforcement, the numbers were low. This year there were 6 regulatory action letters issued by OPDP, the same as the previous year. The only other time in the past 24 years when enforcement was this low was in 2017. This year there two were Warning Letters and four were Untitled Letters.
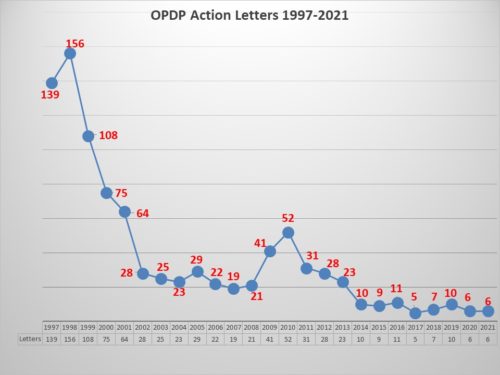
The 6 letters issued involved 7 different communications vehicles for a total of 13 violations that were cited by the agency as reasons for the action.
The Substance: What triggered the enforcement actions? Back in 2009 you may recall that FDA issued 45 Untitled Letters aimed at 14 companies regarding the use of banner ads/sponsored links where clearly benefits of a branded product were available, but risk information only ascertainable by clicking on a link. This was a clear direction from the agency that risk information could not be incorporated by link. This year, three of the communications vehicles involved in enforcement actions were either banner ads or sponsored links where risk information was not included. One of them involved the more serious Warning Letter action, possibly because the product associated with that communication had a Boxed Warning in its label. The other communications vehicles involved DTC videos. Of the 9 violations that were cited in the 5 letters, 4 were for risk minimization or omission, 2 involved superiority claims, 1 unsubstantiated claim and 2 failure to submit Form 2253 to review.
Guidance Docs: As far as I can see, there have been no new relevant guidance documents, wither in draft or final form, that are focused on digital and/or social media and promotional communications. In the latest published guidance agenda from the Center for Drug Evaluation and Research, the advertising and promotion category had no slated guidance efforts listed.
Research. To a degree OPDP signals where it is headed in terms of enforcement and guidance by telling us what research it is conducting around promotional communications. Of the 13 projects currently underway by OPDP, 2 of them are focused on aspects of television DTC ads. There is one study comparing physician interpretation of information from scientific publications versus promotional materials. And curiously, one study is focused on assessing medical conference attendee observations about prescription drug promotion even though medical conferences have largely been virtual for the past two years. None of the studies underway are aimed at uncovering data that would be helpful in providing additional guidance related to the use of digital and social platforms by patients, prescribers and pharma companies.
Why This is a Fail. The way that patients and prescribers learn about medicine has changed significantly over the past 15 years. People do still watch commercial television, but in fewer and fewer numbers. Digital and social media are a primary resource for getting healthcare information and discussing treatment, especially now in the age of COVID. It is a continually evolving environment – there have even been an abundance of articles about how pharma companies should be using TikTok. In particular, for people who do not have access to computers, mobile access is the primary means of getting information. How websites are optimized for mobile is therefore important. FDA has traditionally taken the view that if it is wrong on television DTC, then it is wrong on TikTok. But these are all new platforms that have a lot of nuance to the use of the platforms, in addition to the fact that different populations may have different approaches to digital consumption as well as varied skills. There may be vast comprehension differences in digesting communications from digital sources than from traditional DTC television advertising. It is way past time for the agency to have applied effort to understand the role of that nuance and to make more detailed studies – and therefore guidance – on the best uses of digital media. And of course, there is the fact that FDA never really finished providing guidance related to social media inaugurated by the public meeting the agency held in Washington, D.C. in November 2009. At that meeting, the agency posed five questions as a framework for developing a guidance the following year. Guidance did not come for five years and then did not follow the framework, leaving the questions only partially addressed by two guidance documents – one on use of platforms with character space limitations and another on correcting misinformation posted by third parties. This leaves quite a bit of work not done as it relates to social and digital media, the source from which growing numbers of people get health care information.
FDA Changes Display of Warning/Untitled Letters. Finally, FDA has made it harder to get a view of the regulatory actions with respect to promotion. All letters, Warning and Untitled Letters used to be listed in chronological order. This year, for reasons that are incomprehensible, FDA moved the Warning Letters from the list of OPDP actions and lumped them into all of the other warning letters issued by the entire agency. Now to assess the activity of OPDP for the year, one must visit the list of Untitled Letters and then sort through the list of agency-wide warning letters.
One thing that did happen this year is that OPDP has new leadership. It takes a long time to steer any ships within an agency as large as FDA and to bring about true change. The gears grind slowly. But there is a yawning gap between communications realities on the one hand and the agency focus when it comes to promotional communications. One hopes that the agency will consider the shifts in communications that have taken place and the impact those shifts have on the decisions that patients and providers make with respect to treatment. Hopefully 2022 will bring greater attention to addressing the many deficits that exist in the current approach to enforcement by conducting more research that is appropriate to the communications environment.
Note: Following the original publication of this posting, in mid-January OPDP posted a letter dated December 19. The original of this blog posting has been updated to reflect that late addition to 2021 letters.

