There Are New FDA Safety Warnings About Breast Implants. Here’s What We Know
AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 27, 2022
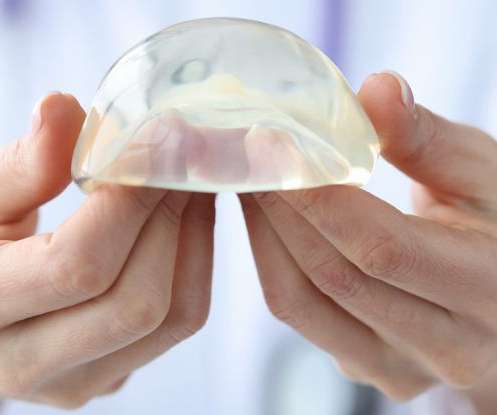
The US Food and Drug Administration (FDA) is now warning that breast implants filled with either silicone or saline may, on rare occasions, give rise to cancer in the scar tissue around breast implants. The safety notice was published this month and is based on an extensive review of the emerging literature on breast implants. […].




































Let's personalize your content