Fourth trial volunteer dies in Astellas gene therapy study
Bio Pharma Dive
SEPTEMBER 14, 2021
The study, which was suspended following three deaths last year, had been restarted in February after Astellas lowered the treatment dose used.

Bio Pharma Dive
SEPTEMBER 14, 2021
The study, which was suspended following three deaths last year, had been restarted in February after Astellas lowered the treatment dose used.
World of DTC Marketing
SEPTEMBER 14, 2021
SUMMARY: Current evidence does not, therefore, appear to show a need for boosting in the general population, in which efficacy against severe disease remains high. Even if humoral immunity appears to wane, reductions in neutralizing antibody titre do not necessarily predict reductions in vaccine efficacy over time, and reductions in vaccine efficacy against mild disease do not necessarily predict reductions in the (typically higher) efficacy against severe disease.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
SEPTEMBER 14, 2021
The venture firm, which previously invested in Disarm Therapeutics, Ra Pharma and Tizona Therapeutics, said its third fund surpassed its target.
Drug Patent Watch
SEPTEMBER 14, 2021
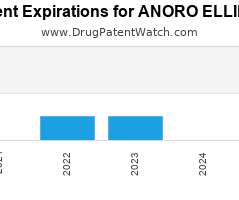
Annual Drug Patent Expirations for ANORO+ELLIPTA Anoro Ellipta is a drug marketed by Glaxosmithkline and is included in one NDA. It is available from one supplier. There are fourteen patents…. The post New patent for Glaxosmithkline drug ANORO ELLIPTA appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Bio Pharma Dive
SEPTEMBER 14, 2021
The German biotech, which announced disappointing results for its shot in June, said the move was done in response to reduced demand.
Drug Patent Watch
SEPTEMBER 14, 2021
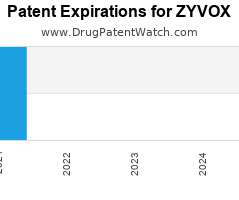
Annual Drug Patent Expirations for ZYVOX Zyvox is a drug marketed by Pfizer and is included in three NDAs. It is available from four suppliers. There are two patents protecting…. The post New patent expiration for Pfizer drug ZYVOX appeared first on DrugPatentWatch - Make Better Decisions.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Outsourcing Pharma
SEPTEMBER 14, 2021
The pharma tech firm has forged a four-year partnership with the foundation to develop antivirals for COVID-19 and other viruses with pandemic potential.

pharmaphorum
SEPTEMBER 14, 2021
There has been a fourth patient fatality in Astellas’ clinical trial of its AT132 gene therapy for the rare disease X-linked myotubular myopathy (XLMTM), which has been halted twice due to safety concerns. The latest suspension came after abnormal liver function tests (LFTs) were seen in a boy receiving the gene therapy, who has since passed away, according to a statement issued today by the Japanese drugmaker.

XTalks
SEPTEMBER 14, 2021
Teatis, a New-York based company, is the maker of superfood powders for diabetic consumers. They announced the close of their seed funding round last week, during which they raised $700,000, bringing the company’s total funding of $1 million. Hiroshi Takatoh is the entrepreneur behind this brand. Takatoh noticed that there is a need for convenient and nutritious food for critically ill consumers.
BioPharma Reporter
SEPTEMBER 14, 2021
CureVac is streamlining its external European manufacturing network for its mRNA product pipeline; having reassessed demand for its first-generation COVID-19 vaccine candidate.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

BioSpace
SEPTEMBER 14, 2021
?According to the study, people who get sick enough to be hospitalized due to the virus might eventually suffer from a full-blown autoimmune disease later on.

pharmaphorum
SEPTEMBER 14, 2021
If there was an AI solution in the Life Sciences field, able to represent a real opportunity in terms of Customer Engagement and Digital Transformation, and if some of the most important players in the industry already had it, would you like to have the opportunity to listen to them? . . This and other trend topics, will be discussed on September 29th , at the digital event “ AI as Business Leverage in Pharma & Life Sciences Industries ” by Trueblue , an Italian IT company that

BioSpace
SEPTEMBER 14, 2021
The company announced a Phase III cardiac drug failed to meet its primary endpoint and a significant shift in its R&D efforts that have resulted in the termination of 75% of its staff.
BioPharma Reporter
SEPTEMBER 14, 2021
Johnson & Johnson says data published in The Lancet on its Ebola vaccine regimen shows it has a robust and durable immune response in adults and children.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

BioSpace
SEPTEMBER 14, 2021
Amylyx’s decision to submit the NDA, announced Wednesday morning, came on the heels of recent discussions with the FDA, including a pre-NDA meeting held on July 15.
Outsourcing Pharma
SEPTEMBER 14, 2021
A survey commissioned by Bristol Myers Squibb reveals healthcare providers are confident that immunotherapy can positively impact earlier-stage cancers.

BioSpace
SEPTEMBER 14, 2021
After multiple attempts, the setup successfully translated signals from his brain to the vocal tract in the form of words that appear as text on a screen.

Pharma Times
SEPTEMBER 14, 2021
Phase Ib trial is evaluating the potential benefit of low-dose psilocybin as a treatment for SUNHA

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
BioSpace
SEPTEMBER 14, 2021
Data coming in about the COVID-19 pandemic shows some promising overall trends, with other more disturbing ones, particularly in breakthrough infections of the fully vaccinated.
Pharma Times
SEPTEMBER 14, 2021
Children in this age group will be offered one dose of the Pfizer/BioNTech jab from next week

BioSpace
SEPTEMBER 14, 2021
The U.S. FDA has been actively greenlighting a number of efforts over the last few days to push therapies for rare or serious diseases that have largely unmet medical needs. Here's a look.

Pharma Times
SEPTEMBER 14, 2021
Platform launched in a bid to help life sciences companies address the insights gap

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
BioSpace
SEPTEMBER 14, 2021
The European Society for Medical Oncology Congress 2021 runs from September 16–21, 2021. Here’s a preview of some of the presentations at the meeting.
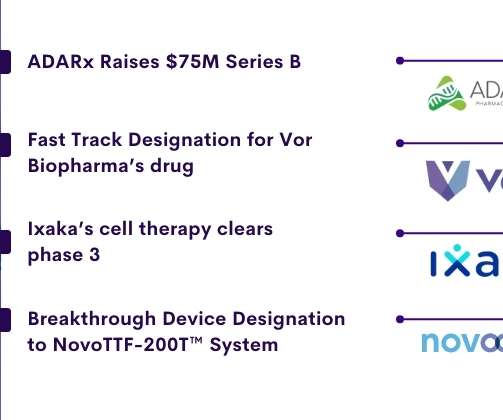
Delveinsight
SEPTEMBER 14, 2021
ADARx bags USD 75 Million to advance its RNA tech pipeline. ADARx Pharmaceuticals, a biotechnology company developing RNA targeting therapeutics , announced the completion of a USD 75 million Series B financing to progress its drug development pipeline. SR One Capital Management and OrbiMed Advisors co-led Series B. Sirona Capital united this financing as well as existing investors OrbiMed Advisors and Lilly Asia Ventures.

BioSpace
SEPTEMBER 14, 2021
The mobile app, dubbed Brisa, has been designed to help patients record their disease progression regardless of condition and treatment plans.

Drug Channels
SEPTEMBER 14, 2021
On October 5, 2021, Drug Channels Institute will release The 2021–22 Economic Report on Pharmaceutical Wholesalers and Specialty Distributors. This report—our twelfth edition— remains the most comprehensive, fact-based tool for understanding and analyzing the large and growing U.S. pharmaceutical distribution industry. We are providing you with the opportunity to preorder this thoroughly updated and revised 2021-22 edition at special discounted prices.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

BioSpace
SEPTEMBER 14, 2021
Sanofi is shuttering Principia’s laboratories in San Francisco after its Phase III PEGASUS trial of rilzabrutinib for pemphigus failed to meet primary or key secondary endpoints.

Outsourcing Pharma
SEPTEMBER 14, 2021
A physician assistant from Hawthorne Effect shares how she and other trained health professionals contribute their knowledge and talents to in-home studies.

BioSpace
SEPTEMBER 14, 2021
Life sciences companies reeled in millions of dollars in Series C financing rounds this week. Here is a look at a few of those companies and what their fresh funds will be used for.
Scienmag
SEPTEMBER 14, 2021
The Texas Heart Institute has announced that a new study from its prolific Cardiomyocyte Renewal Lab has been published in Circulation Research – a peer-reviewed journal from the American Heart Association that reaches clinical and academic cardiologists, basic cardiovascular scientists, physiologists, cellular and molecular biologists, and cardiovascular pharmacologists.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content