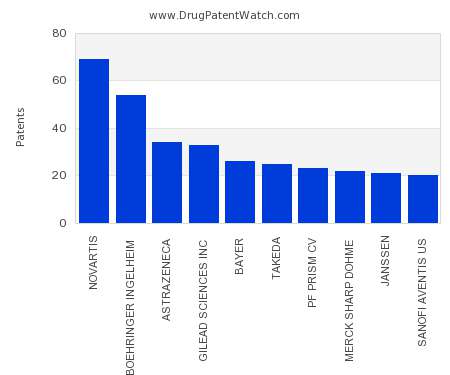
Top Novartis executives to depart as company restructures
Bio Pharma Dive
APRIL 4, 2022
The Swiss pharma said its plan to merge multiple business units and cut yearly costs by $1 billion is likely to impact jobs, but declined to specify which roles and how many.



































Let's personalize your content