EMA CHMP recommends Moderna’s Covid-19 booster for children
Pharmaceutical Technology
DECEMBER 18, 2022
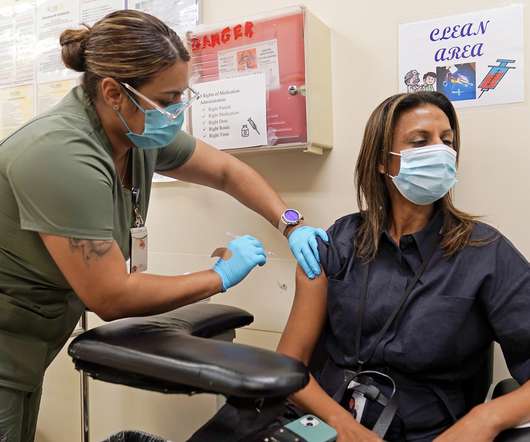
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended granting variation to the marketing authorization (MA) for Moderna ’s Spikevax bivalent Original/Omicron BA.1 (mRNA-1273.214) booster for usage in children aged six to 11 years. The 0.25mL dose of the booster vaccine could potentially be used in the European Union (EU) following authorisation in these children a minimum of three months following a previous Covid-19 vaccination.
























Let's personalize your content