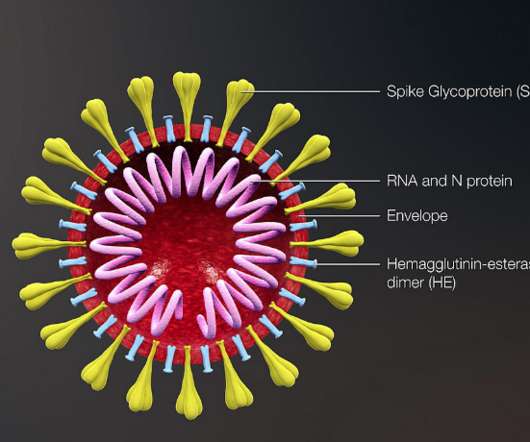
AstraZeneca, Oxford coronavirus vaccine prevents COVID-19, though study results raise questions
Bio Pharma Dive
NOVEMBER 23, 2020
Data from two late-stage trials showed the shot to be, on average, 70% effective against COVID-19, an encouraging finding. The lower dose tested, however, outperformed a higher dose.































Let's personalize your content