Roche hands rights to lung cancer drug back to Blueprint
Bio Pharma Dive
FEBRUARY 24, 2023
Weeks after the Swiss drugmaker wrote off Gavreto’s accounting value, the biotech will regain responsibility for its commercialization.

Bio Pharma Dive
FEBRUARY 24, 2023
Weeks after the Swiss drugmaker wrote off Gavreto’s accounting value, the biotech will regain responsibility for its commercialization.

Pharmaceutical Technology
FEBRUARY 24, 2023
(LAI-287 + semaglutide) is under clinical development by Novo Nordisk and currently in Phase III for Type 2 Diabetes. According to GlobalData, Phase III drugs for Type 2 Diabetes have a 53% phase transition success rate (PTSR) indication benchmark for progressing into Pre-Registration. GlobalData’s report assesses how (LAI-287 + semaglutide)’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
FEBRUARY 24, 2023
The EMA's drug committee concluded a clinical benefit “could not be demonstrated” for the antiviral pill Lagevrio, a decision Merck and partner Ridgeback said they will appeal.

Pharmaceutical Technology
FEBRUARY 24, 2023
The EU is planning a sweeping revision of its pharma legislation in March, the largest change in 20 years. The overhaul will address drug marketing exclusivity length, pricing, patient access, innovation incentives, antimicrobial resistance, clinical trials, supply chain security and shortages, and environmental impact. The revision process is reviving old conflicts in the pharma industry over fair and affordable access to medicines versus Europe’s status as a competitive home for pharma innovat

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Rethinking Clinical Trials
FEBRUARY 24, 2023
The National Institute on Drug Abuse (NIDA) invites primary care clinical and research teams to take part in a new Challenge competition funding opportunity, “Substance Use Prevention Services in Primary Care!” With this Challenge, NIDA is seeking better understanding of how primary care providers can implement substance use prevention interventions in their clinical settings.

Pharmaceutical Technology
FEBRUARY 24, 2023
Sanofi has received approval from the US Food and Drug Administration (FDA) for its Altuviiio [Antihemophilic Factor (Recombinant) , Fc-VWF-XTEN Fusion Protein-ehtl], to treat a type of inherited bleeding disorder known as haemophilia A. Altuviiio, previously referred to as efanesoctocog alfa, is indicated for routine prophylaxis and on-demand treatment for controlling bleeding episodes and perioperative management (surgery) for haemophilia A adults and children.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Pharmaceutical Technology
FEBRUARY 24, 2023
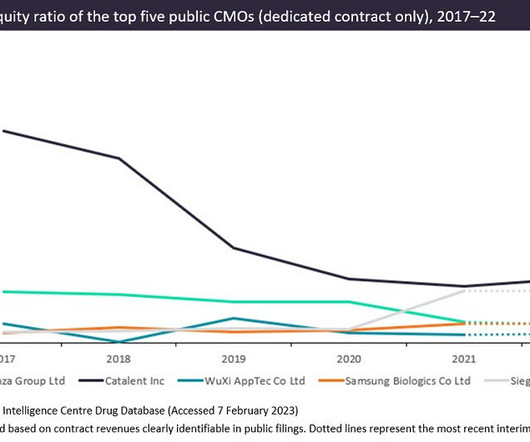
Pharma manufacturers will likely be unable to invest as much in innovative capabilities as they did previously due to the current environment of high inflation and quickly rising interest rates. Instead, many manufacturers will refocus on operational efficiency in the coming months. During a time when increasingly innovative biologics are being approved and pharma companies are seeking contractors to mitigate the risk of supply chain disruptions, the long-term effects of slowing investment could

Bio Pharma Dive
FEBRUARY 24, 2023
Part of a plan to lower spending, the workforce reduction will involve layoffs as well as leaving positions unfilled after employees depart.
Pharmaceutical Technology
FEBRUARY 24, 2023
As the Crohn’s disease (CD) therapeutic landscape grows crowded with added therapies, the success of new agents entering the market depends on their ability to show distinction from comparable first-in-class drugs. This point is illustrated by Galapagos’s recent announcement that Jyseleca (filgotinib maleate), the Janus kinase inhibitor (JAKi) being evaluated as a treatment for adults with moderate to severe CD, did not meet its co-primary endpoint of clinical remission and endoscopic response i

Medical Xpress
FEBRUARY 24, 2023
Researchers from the University of Cincinnati examined the post-treatment journals kept by participants in a 2014 smoking cessation study that found psychedelics were effective in helping some people quit smoking for years.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
FEBRUARY 24, 2023
Retatrutide is a biologic commercialized by Eli Lilly and Co, with a leading Phase II program in Obesity. According to Globaldata, it is involved in 10 clinical trials, of which 4 were completed, 5 are ongoing, and 1 is planned. GlobalData uses proprietary data and analytics to provide a complete picture of Retatrutide’s valuation in its risk-adjusted NPV model (rNPV).

STAT News
FEBRUARY 24, 2023
A classic warning in public health goes like this: “A society that spends so much on health care that it cannot or will not spend adequately on other health enhancing activities may actually be reducing the health of its population.” No nation is as guilty of this practice as the United States, with its extremely high health expenditures alongside abysmal population-level health outcomes.

Pharmaceutical Technology
FEBRUARY 24, 2023
(LAI-287 + semaglutide)is under clinical development by Novo Nordisk and currently in Phase III for Type 2 Diabetes.

STAT News
FEBRUARY 24, 2023
As a health policy wonk and health economist who has worked in pharmaceutical companies in the United States, Latin America, and Europe, I’ve seen vast volumes of data generated, gathered, aggregated, analyzed, shared, and resold by health care companies and organizations. In my studies with the world’s top medical statistics experts at the University of Oxford’s Centre for Evidence Based Medicine, I’ve also seen how flawed many datasets are, missing critical data pie

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharmaceutical Technology
FEBRUARY 24, 2023
ACE-1334 is under clinical development by Merck and currently in Phase I for Lung Disease. According to GlobalData, Phase I drugs for Lung Disease have a 92% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData’s report assesses how ACE-1334’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks.

Fierce Pharma
FEBRUARY 24, 2023
Moderna pays US government $400M 'catch-up payment' under new COVID-19 vaccine license esagonowsky Fri, 02/24/2023 - 09:18

Pharmaceutical Technology
FEBRUARY 24, 2023
(Lopinavir + ritonavir) is under clinical development by Douglas Pharmaceuticals and currently in Phase I for Genital Warts (Condylomata Acuminata). According to GlobalData, Phase I drugs for Genital Warts (Condylomata Acuminata) have a 91% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData’s report assesses how (Lopinavir + ritonavir)’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks.
NPR Health - Shots
FEBRUARY 24, 2023
Sure, you may resent how much of your energy gets sucked up by your job. But research finds that keeping up relationships with colleagues may have a big upside to your health and happiness.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
FEBRUARY 24, 2023
Amlitelimab is under clinical development by Kymab and currently in Phase II for Asthma. According to GlobalData, Phase II drugs for Asthma have a 27% phase transition success rate (PTSR) indication benchmark for progressing into Phase III. GlobalData’s report assesses how Amlitelimab’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks.

STAT News
FEBRUARY 24, 2023
The National Institutes of Health provided $362 million in grants for clinical trials that enrolled at least 41,000 children over a recent three-year period — but many results were never published, a new analysis found. The results are a worrisome sign of a lack of transparency that can lead to wasted research funding. Specifically, just under two-thirds of the 354 trials studied were registered in advance with ClinicalTrials.gov , the federal database, and just 13% of finished trials wer

Pharmaceutical Technology
FEBRUARY 24, 2023
ACD-440 is under clinical development by AlzeCure Pharma and currently in Phase I for Neuropathic Pain (Neuralgia). According to GlobalData, Phase I drugs for Neuropathic Pain (Neuralgia) have a 77% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData’s report assesses how ACD-440’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks.

STAT News
FEBRUARY 24, 2023
WASHINGTON — The head of the FDA’s tobacco center wants to do more to tout the health benefits of switching from cigarettes to e-cigarettes, now that youth vaping rates are declining. “With the reductions in [youth vaping rates] that we’ve seen, we’ve got an opportunity to ramp up our efforts related to the continuum of risk,” said Brian King, director of the FDA’s Center for Tobacco Products, at a Friday event.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

Pharmaceutical Technology
FEBRUARY 24, 2023
(Lopinavir + ritonavir) is under clinical development by Douglas Pharmaceuticals and currently in Phase I for Cervical Intraepithelial Neoplasia (CIN). According to GlobalData, Phase I drugs for Cervical Intraepithelial Neoplasia (CIN) have a 92% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData’s report assesses how (Lopinavir + ritonavir)’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmar
Medical Xpress
FEBRUARY 24, 2023
Researchers from the University of Queensland have identified that a gene associated with an increased risk of Parkinson's disease also contributes to a build-up of cell debris in the brain.

Pharmaceutical Technology
FEBRUARY 24, 2023
Inaxaplin is a small molecule commercialized by Vertex Pharmaceuticals, with a leading Phase III program in Focal Segmental Glomerulosclerosis (FSGS). According to Globaldata, it is involved in 3 clinical trials, of which 2 were completed, and 1 is ongoing. GlobalData uses proprietary data and analytics to provide a complete picture of Inaxaplin’s valuation in its risk-adjusted NPV model (rNPV).
Medical Xpress
FEBRUARY 24, 2023
A study conducted by researchers at the University of Liège on group 2 innate lymphoid cells (or ILC2s) shows that the functional reprogramming of these cells following their exposure to viruses allows our body to react differently to exposure to certain respiratory allergens. This study is published in Science Immunology.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharmaceutical Technology
FEBRUARY 24, 2023
Amivantamab is under clinical development by Johnson & Johnson and currently in Phase I for Colorectal Cancer. According to GlobalData, Phase I drugs for Colorectal Cancer have a 76% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData’s report assesses how Amivantamab’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks.

STAT News
FEBRUARY 24, 2023
In the latest flare up over access to the abortion pill, a dozen states filed a lawsuit to force the U.S. Food and Drug Administration to ease restrictions on how mifepristone is prescribed and made available to patients. At issue is a risk mitigation program, which is used to ensure certain prescription medicines considered to carry significant risks are prescribed and taken safely.

Pharmaceutical Technology
FEBRUARY 24, 2023
Inaxaplin is a small molecule commercialized by Vertex Pharmaceuticals, with a leading Phase III program in Focal Segmental Glomerulosclerosis (FSGS).

STAT News
FEBRUARY 24, 2023
A looming Texas court decision on abortion pills could impact nationwide access to medication, Vice President Kamala Harris warned Friday, as she described abortion access as a “constitutional right.” The decision — which could be handed down as early as Friday — could yank mifepristone off the market more than two decades after the pill, also used to treat miscarriage, was approved by the Food and Drug Administration.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content