Novartis joins Sanofi in aiding manufacture of coronavirus vaccines
Bio Pharma Dive
JANUARY 29, 2021
A deal to fill vials of Pfizer and BioNTech's vaccine could be the first of several Novartis signs to help others make COVID-19 treatments.

Bio Pharma Dive
JANUARY 29, 2021
A deal to fill vials of Pfizer and BioNTech's vaccine could be the first of several Novartis signs to help others make COVID-19 treatments.

World of DTC Marketing
JANUARY 29, 2021
SUMMARY: The future of healthcare involves digital transformation but moving too fast in a matrix management environment can cause problems and set digital back months. Most of us know the importance of digital when to comes to healthcare. The pandemic has accelerated digital transformation, but organizations need to be careful about moving too fast without getting company influencers’ buy-in.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
JANUARY 29, 2021
Investors feared an outright rejection for aducanumab. But the FDA moving its approval deadline back three months is a signal to some that the agency wants to approve Biogen's drug.

pharmaphorum
JANUARY 29, 2021
Genome editing is an exciting but still nascent field, and companies in the area face as many obstacles as they do opportunities. Sangamo CEO Sandy Macrae told us how his company is being cautious about the hype and finding ways to be financially viable in an emerging space. ‘Cutting edge’ is, for once, a truly apt description when it comes to gene editing – both because the field is pushing medicine into areas we might never have dreamed possible, and because these technologies involve literall

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

XTalks
JANUARY 29, 2021
Oncology products monopolise the list of best-selling drugs. With a huge range of indications that is only growing, therapies become more specific to tumour or cell pathologies and are suitable for an increasing number of patients. Several oncology products now have “blockbuster” status where sales exceed $1 billion annually. This is relevant for clinical trials as more regulators require evidence of efficacy in comparison to the standard of care, which is likely to be one of the blockbuster pro
pharmaphorum
JANUARY 29, 2021
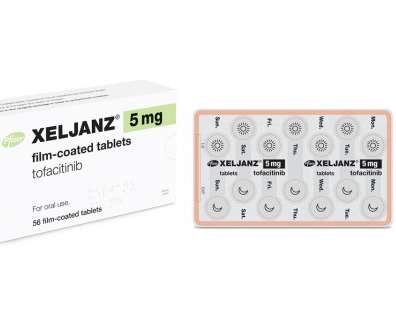
JAK inhibitor Xeljanz is one of Pfizer’s top-selling drugs, despite a ‘black box’ warning for blood clots and cancers added to its label in 2019. Now, a study designed to prove its safety has achieved the opposite. The post-marketing safety study showed that Xeljanz (tofacitinib) – used to treat rheumatoid arthritis (RA) – was associated with a higher rate of heart attacks and cancer than a TNF inhibitor in patients aged over 50 with underlying cardiovascular risk factors.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
pharmaphorum
JANUARY 29, 2021
Novavax’s COVID-19 vaccine could be added to the UK’s growing arsenal of shots against the disease after it showed 89.3% efficacy in a late-stage trial. The shot is the first to show efficacy against the new variant found in the UK which accounted for around half of cases in the phase 3 study. Efficacy against the original COVID-19 strain was calculated at around 95.6%, falling to to around 85.6% against the UK variant strain, based on a post-hoc analysis.
BioPharma Reporter
JANUARY 29, 2021
Novavaxâs COVID-19 vaccine showed 89.3% efficacy in its Phase 3 UK trial: including against the UK variant. Meanwhile a Phase 2b trial suggests 60% efficacy against the South African variant. âOur vaccine is the first to demonstrate significant clinical efficacy against both the rapidly emerging UK and South Africa variants,â says the company.
pharmaphorum
JANUARY 29, 2021
Four million UK patients could benefit annually from genetic testing before being prescribed common medicines, according to new research. Researchers from the University of East Anglia (UEA) in collaboration with Boots UK and Leiden University analysed 2019 NHS dispensing data across the UK. The goal was to see how many patients are started on new prescriptions each year that could be potentially optimised by genetic testing.

BioPharma Reporter
JANUARY 29, 2021
The EU Commission has published a redacted version of the contract it signed with AstraZeneca on COVID-19 vaccine delivery in August last year, in a bid to show that the pharma giant is backtracking on its commitments.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
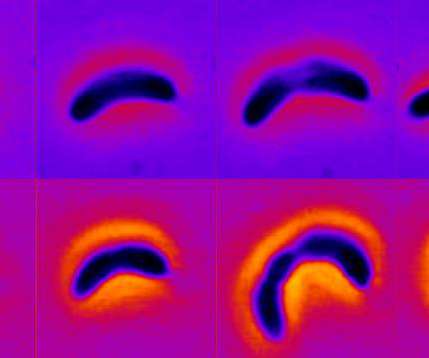
Scienmag
JANUARY 29, 2021
Credit: Shiladitya Banerjee New research led by Carnegie Mellon University Assistant Professor of Physics Shiladitya Banerjee demonstrates how certain types of bacteria can adapt to long-term exposure to antibiotics by changing their shape. The work was published this month in the journal Nature Physics. Adaptation is a fundamental biological process driving organisms to change their […].

Pharma Times
JANUARY 29, 2021
Datopotamab deruxtecan and Enhertu were evaluated in non-small cell lung cancer patients
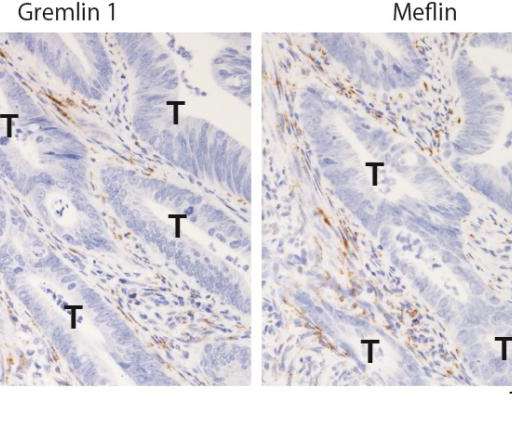
Scienmag
JANUARY 29, 2021
Credit: Atsushi Enomoto Nagoya University researchers and colleagues have revealed that colorectal cancer tissues contain at least two types of fibroblasts (a type of cells found in connective tissue), namely, cancer-promoting fibroblasts and cancer-restraining fibroblasts, and that the balance between them is largely involved in the progression of colorectal cancer.
Pharma Times
JANUARY 29, 2021
Speciality vaccine company has also started production of vaccine candidate

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Scienmag
JANUARY 29, 2021
Credit: Diana Kenney WOODS HOLE, Mass. — The most powerful substance in the human brain for neuronal communication is glutamate. It is by far the most abundant, and it’s implicated in all kinds of operations. Among the most amazing is the slow restructuring of neural networks due to learning and memory acquisition, a process called […].
Pharma Times
JANUARY 29, 2021
Meanwhile, Phase IIb results from South Africa trial demonstrated 60% efficacy

Scienmag
JANUARY 29, 2021
Despite best efforts to distribute free meals, study notes was a 58 percent drop in number of meals provided to children in need Credit: University of Maryland School of Medicine School closures during COVID-19 have decreased access to school meals, which is likely to increase the risk for food insecurity among children in Maryland, according […].

Pharma Times
JANUARY 29, 2021
GBT is aiming for the approval of Oxbryta to treat haemolytic anaemia in patients with sickle cell disease

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Scienmag
JANUARY 29, 2021
‘Organs-on-a-chip’ system sheds light on how bacteria in the human digestive tract may influence neurological diseases Credit: Martin Trapecar, MIT CAMBRIDGE, MA — In many ways, our brain and our digestive tract are deeply connected. Feeling nervous may lead to physical pain in the stomach, while hunger signals from the gut make us feel irritable. […].

BioPharma Reporter
JANUARY 29, 2021
The European Medicines Agency (EMA) today advised that AstraZenecaâs COVID-19 vaccine can be given conditional marketing authorization (CMA) in the EU to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older.

Scienmag
JANUARY 29, 2021
Pritzker says IRP is “the type of strategic investment” to move Illinois forward Credit: Michael Schmidt NORTH CHICAGO, ILL. — The $50 million Innovation and Research Park (IRP) on the campus of Rosalind Franklin University of Medicine and Science (RFU) marked its first anniversary with a virtual opening ceremony on Jan. 28, featuring dignitaries and […].

The Pharma Data
JANUARY 29, 2021
HALIFAX, Nova Scotia, Jan. 29, 2021 (GLOBE NEWSWIRE) — Today, MedMira Inc. (MedMira) (TSXV: MIR) announces the development of a rapid antibody test prototype that detects the presence of the neutralizing antibodies against the SARS-CoV-2 virus. These antibodies bind to the specific parts of the virus, decreasing the viral infectivity and potentially protecting the patient from the severe COVID-19 symptom presentation or future SARS-CoV-2 reinfection.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

Scienmag
JANUARY 29, 2021
UC Davis nationwide survey News reports indicate COVID-19 vaccines are not getting out soon enough nor in adequate supplies to most regions, but there may be a larger underlying problem than shortages. A University of California, Davis, study found that more than a third of people nationwide are either unlikely or at least hesitant to […].

The Pharma Data
JANUARY 29, 2021
29 January 2021 — AstraZeneca’s COVID-19 vaccine has been recommended for conditional marketing authorisation (CMA) in the European Union (EU) for active immunisation to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older. Following review of the application, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency based its positive opinion on data from a rolling review of trial data from the primary analysis of the Phase III p

Scienmag
JANUARY 29, 2021
A new report combining forecasting and expert prediction data, predicts that 125,000 lives could be saved by the end of 2021 if 50% or more of the U.S. population initiated COVID vaccination by March 1, 2021. “Meta and consensus forecast of COVID-19 targets,” developed by Thomas McAndrew, a computational scientist and faculty member at Lehigh […].

The Pharma Data
JANUARY 29, 2021
29 January 2021 — AstraZeneca’s COVID-19 vaccine has been granted a conditional marketing authorisation (CMA) in the European Union (EU) for active immunisation to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older. Following review of the application, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency based its positive opinion on data from a rolling review of trial data from the primary analysis of the Phase III program

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Scienmag
JANUARY 29, 2021
Research presented at IASLC 2020 World Conference on Lung Cancer Singapore (Singapore–January 29, 2021 9:35 a.m. SPT/January 28, 2021 8:35 p.m. EST)–Two radiation oncology trials presented at the IALSC World Conference on Lung Cancer Singapore highlight how some researchers are exploring use of higher radiation boost doses to only PET-positive regions in locally-advanced non-small cell […].

The Pharma Data
JANUARY 29, 2021
French vaccine company Valneva said it has begun production of its investigational COVID-19 vaccine, which is now in a phase 1/2 clinical trial in the UK. The study has enrolled 150 adult volunteers for a two-dose inoculation and initial results are expected in April. Valneva also plans to test the vaccine in more than 4,000 patients in additional trials.

Scienmag
JANUARY 29, 2021
In 2017, the Nobel Prize in Physiology or Medicine went to three scientists who uncovered the molecular mechanisms that control the circadian rhythm, otherwise known as the “wake-sleep” cycle. To carry out their work, the scientists used the common fruit fly Drosophila melanogaster, making this the sixth Nobel to be awarded to research involving it. […].

The Pharma Data
JANUARY 29, 2021
FRIDAY, Jan. 29, 2021 (American Heart Association News) — Sam Rafferty fell severely ill after getting infected with COVID-19 at an uncle’s funeral in March, an event that sickened her entire family and left Rafferty and her daughter struggling to breathe. But it’s not her lungs that have kept her from making a full recovery; it’s her brain. “I coughed for two and a half months.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content