The US Food and Drug Administration (FDA) has granted Fast Track designation for Avidity Biosciences’ AOC 1020 to treat facioscapulohumeral muscular dystrophy (FSHD).
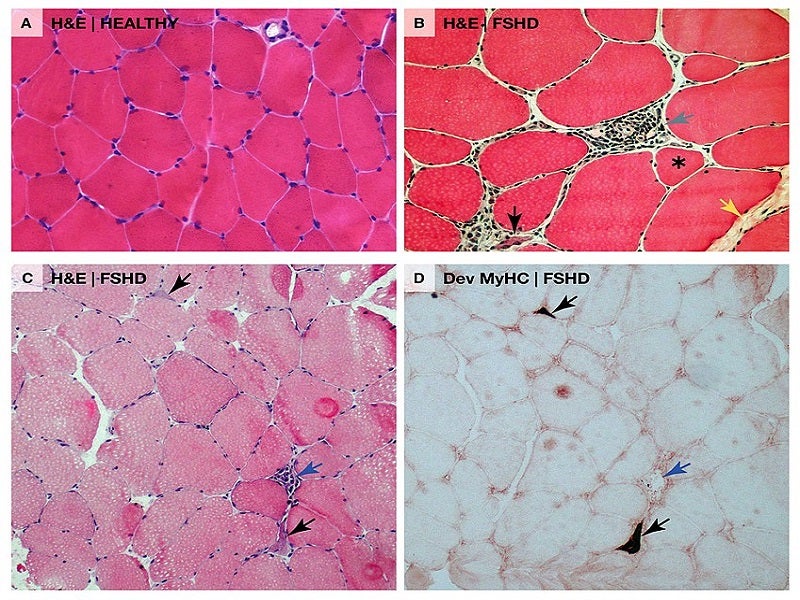
AOC 1020 has been designed for the treatment of the underlying cause of FSHD, which is caused by the abnormal expression of a gene known as double homeobox 4 or DUX4.
This DUX4 protein abnormal expression leads to modifications in gene expression in muscle cells which are associated with progressive muscle function loss in FSHD patients.
Avidity Biosciences’ AOC 1020 is intended to reduce the DUX4 mRNA and DUX4 protein expression in muscles in these patients.
It comprises a monoclonal antibody (mAb) attached to the transferrin receptor 1 (TfR1) conjugated with a DUX4 mRNA that targets siRNA.
See Also:
Avidity Biosciences chief medical officer Steve Hughes said: “The FDA Fast Track designation for AOC 1020 reinforces the importance of finding an effective treatment to help people living with FSHD, a devastating and debilitating muscular dystrophy disorder with no treatment options.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData“AOC 1020 is designed to directly target the disease-causing gene, DUX4, to address the underlying cause of FSHD.
“We look forward to working collaboratively with the FDA to bring the first RNA therapy directly targeting DUX4 to patients as quickly as possible.”
AOC 1020 is currently being studied in the double-blind, randomised, placebo-controlled Phase I/II FORTITUDE clinical trial in nearly 70 FSHD adult patients.
The trial has been designed to assess the pharmacokinetics, safety, pharmacodynamics, and tolerability of AOC 1020, which is intravenously given to patients.
The company intends to share the data from a preliminary assessment of AOC 1020 with nearly half of the participants in the first half of next year.




