5 questions facing emerging biotech in 2023
Bio Pharma Dive
JANUARY 10, 2023
Investors expect another lackluster year in IPOs, while company restructurings look set to continue as biotech executives try to conserve cash.

Bio Pharma Dive
JANUARY 10, 2023
Investors expect another lackluster year in IPOs, while company restructurings look set to continue as biotech executives try to conserve cash.
Pharmaceutical Technology
JANUARY 13, 2023
Health Canada has granted approval to Enhertu (trastuzumab deruxtecan) to treat unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer. Enhertu has been approved to treat HER2-low breast cancer adult patients who have previously received at least one line of chemotherapy in the metastatic setting or who have seen disease recurrence during or within six months after the adjuvant chemotherapy.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 12, 2023
COVID-19 is defined as a respiratory infection, but the effects of the novel coronavirus are certainly not confined to any one organ. Dozens of recent autopsies show persistent evidence of SARS-CoV-2 throughout the body, including in the lungs, the heart, the spleen, the kidneys, the liver, the colon, the thorax, muscles, nerves, the reproductive tract, […].
STAT News
JANUARY 12, 2023
The percentage of U.S. kindergartners who’ve received standard childhood vaccines took a small but notable dip into the 2021-2022 school year, health officials said Thursday, amid disruptions related to Covid-19 and fears that anti-vaccine sentiment stirred up by the pandemic could be spreading to other shots. Vaccinations among children remain high, but the trend — with coverage dropping from about 95% in the 2019-2020 school year to 94% in 2020-2021 to 93% in 2021-2022, according

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Bio Pharma Dive
JANUARY 9, 2023
An Alzheimer’s drug from Eli Lilly, BioMarin’s hemophilia gene therapy and a new type of breast cancer treatment are among the top decisions on the agency’s docket through the end of March.

Pharmaceutical Technology
JANUARY 8, 2023
In 2023, the pharmaceutical industry will mark 20 years since Xolair, an anti-IgE antibody, became the first biologic approved to treat asthma. Since then, the US FDA, EMA, and other agencies have approved several biologic antibodies targeting the inflammatory cytokines IL-4, IL-13, IL-5, and others for asthma. Approaches like bronchodilator inhalers focus on treating both asthma and chronic obstructive pulmonary disease (COPD).
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
STAT News
JANUARY 12, 2023
Ready or not, health care is undergoing a massive transformation driven by artificial intelligence. But medical schools have barely started to teach about AI and machine learning — creating knowledge gaps that could compound the damage caused by flawed algorithms and biased decision-support systems. “We’re going to be at a point where we’re not going to be able to catch up and be able to call out the technology defects or flaws,” said Erkin Ötleş, a

Bio Pharma Dive
JANUARY 10, 2023
The FDA commissioner shared advice for drugmakers at the J.P. Morgan Healthcare conference, while venture investors cautioned of a coming funding crunch for young biotechs.

Pharmaceutical Technology
JANUARY 9, 2023
Eisai and Biogen have received approval for their antibody Leqembi (lecanemab-irmb) , 100mg/mL injection for intravenous use, from the US Food and Drug Administration (FDA) under the Accelerated Approval Pathway to treat Alzheimer’s disease (AD). Leqembi is indicated to treat mild cognitive impairment or mild dementia stage of the disease in patients whose treatment started in clinical trials.
AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 10, 2023
The US Food and Drug Administration (FDA) on Friday approved a highly anticipated new drug designed to slow cognitive decline in patients in mild and early stages of Alzheimer’s disease. The FDA approval of the drug, Leqembi, also known as lecanemab, comes just days after the regulatory agency was harshly criticized in a congressional report […].

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

STAT News
JANUARY 7, 2023
Joan Cohrs missed her chance to grab a prescription at her usual drugstore by about 30 seconds. Cohrs walked up to the counter of an Indianapolis CVS pharmacy shortly after a metal curtain descended, closing it for lunch. She didn’t mind. The 60-year-old said she feels compassion for people in health care.

Bio Pharma Dive
JANUARY 11, 2023
Executives at the two pharmas talked up their rival autoimmune disease drugs at the J.P. Morgan Healthcare Conference, while Prime Medicine’s CEO pondered a busy future.

Pharmaceutical Technology
JANUARY 10, 2023
AstraZeneca has signed a definitive agreement to buy US-based biopharmaceutical company CinCor Pharma in a deal valued at about $1.8bn. Under the deal, the company will initiate a tender offer to purchase all the outstanding shares of CinCor Pharma for $26 per share in cash. The transaction also includes a non-tradable contingent value right of $10 per share in cash which will be paid after a specified regulatory submission of CinCor’s lead drug candidate, baxdrostat (CIN-107).

NPR Health - Shots
JANUARY 11, 2023
When first lady Jill Biden went for routine surgery for a small lesion above her right eye, doctors found two more lesions, and removed them, too, the White House said.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

STAT News
JANUARY 13, 2023
Oscar Health has excelled at one thing in particular since its inception a decade ago: burning cash. The health insurance company did not plan to be profitable immediately. It was staking its future on the new Affordable Care Act marketplaces and had to grow quickly to compete with larger insurance carriers. Oscar organized its health insurance product through an app, promoted virtual care, advertised on New York subways, and then low-balled prices in certain markets to attract young, healthy pe

Bio Pharma Dive
JANUARY 9, 2023
Drug price reforms and regulatory scrutiny present challenges for large drugmakers, although successes in Alzheimer’s and obesity have brought new opportunities, too.
Medical Xpress
JANUARY 12, 2023
Psychiatric illnesses, such as schizophrenia and depression, affect nearly one in five adults in the United States and nearly half of patients diagnosed with a psychiatric illness also meet the criteria for a second. With so much overlap, researchers have begun to suspect that there may be one neurobiological explanation for a variety of psychiatric illnesses.

NPR Health - Shots
JANUARY 12, 2023
In a victory for animal rights advocates, drugmakers can take their products to human clinical trials using alternative testing methods that don't involve animals.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
STAT News
JANUARY 10, 2023
Mistakes happen — in life, in the lab, and, inevitably, in research papers, too. Journals use corrections and retractions to resolve those mistakes. But one particularly high-profile case is now drawing fresh attention to the problems with journals’ process for addressing concerns about research integrity. Late last year, Stanford University announced that it was opening an investigation into its president, neuroscientist Marc Tessier-Lavigne, over allegations of research misconduc
Bio Pharma Dive
JANUARY 12, 2023
Buoyed by recent approvals, the field faces a pivotal year that’s likely to bring new treatments as well as more challenges.

BioPharma Reporter
JANUARY 10, 2023
German mRNA pioneer BioNTech will acquire InstaDeep, an artificial intelligence and machine learning specialist, for around Â362m ($439m): pledging to incorporate rapidly evolving AI capabilities into its research, drug discovery, manufacturing and deployment processes.

NPR Health - Shots
JANUARY 10, 2023
Donations often come with rigorous applications and reporting requirements. Billionaire MacKenzie Scott, who divorced Jeff Bezos in 2019 and vowed to give away most of her fortune, does it her way.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

STAT News
JANUARY 11, 2023
The air in Mexico City was once so toxic that people watched as dead birds fell out of the sky. In 1992, the United Nations declared the city the most polluted in the world, with its unregulated diesel engines, factory production, fossil-fuel powered energy plants, and widespread use of internal-combustion engines, all trapped in a high-altitude, mountain-lined valley.

Bio Pharma Dive
JANUARY 11, 2023
Airsupra joins a growing portfolio of new drugs that are helping the British drugmaker offset wilting sales of older respiratory medicines like Pulmicort.

pharmaphorum
JANUARY 12, 2023
Bayer has signed an agreement with Google aimed at using high-level processing power to handle quantum chemistry calculations used to predict the chemical and physical properties of drug molecules at the atomic scale. The deal with the tech giant’s Google Cloud unit revolves around its tensor processing units (TPUs), artificial intelligence-powered accelerators designed to run machine learning models and computationally-intensive workloads that can be customised to specific applications.

NPR Health - Shots
JANUARY 8, 2023
The public school district in Seattle has filed a novel lawsuit against TikTok, Instagram, Facebook, YouTube and Snapchat, seeking to hold them accountable for a mental health crisis among youth.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
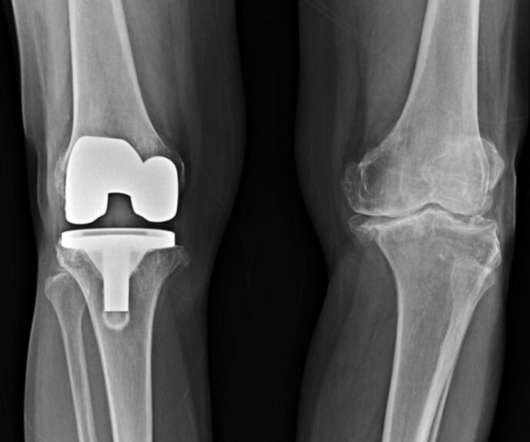
STAT News
JANUARY 10, 2023
The Food and Drug Administration’s approach to evaluating some new medical devices might actually increase the risk that those devices will later be recalled over safety concerns. For some medical devices, the FDA requires data from studies in people to show that the device, including things like implantable heart defibrillators or stents, is safe and effective.

Bio Pharma Dive
JANUARY 11, 2023
The agency will kick off discussions on how it will negotiate Medicare drug prices in the spring and publish a list of the first 10 drugs selected by Sept. 1, 2023.
pharmaphorum
JANUARY 11, 2023
Over the past few decades, data generation has veritably exploded. However, the ‘Big Data paradigm’ is not so much concerned with the volume of that data, but how businesses and, indeed, industries can derive meaningful insights from what has become a glut of information. With the currently popular approach to artificial intelligence (AI) focussing on the Big Data paradigm, also, pharmaphorum spoke with Adityo Prakash, CEO of Verseon, about the whys and wherefores, delving deeper into the proces

NPR Health - Shots
JANUARY 11, 2023
People who lose track of time aren't rude, researchers say — they may just be listening to their inner timekeeper instead of an external clock. Living according to "event time" has its benefits.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Let's personalize your content