Novartis secures FDA approval for Pluvicto production at US plant
Pharmaceutical Technology
JANUARY 8, 2024
Novartis has received the US FDA approval to commercially manufacture Pluvicto at its new RLT manufacturing facility.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
JANUARY 8, 2024
Novartis has received the US FDA approval to commercially manufacture Pluvicto at its new RLT manufacturing facility.
Pharmaceutical Technology
MARCH 8, 2023
It offers an alternative to a product for which there is a shortage. The regulatory approval marks the company’s first product to receive approval in the US market. The post US FDA approves Shorla’s oncology drug for T-cell leukaemia appeared first on Pharmaceutical Technology.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Bio Pharma Dive
NOVEMBER 16, 2023
Acquired via a $4 billion biotech buyout, Augtyro is one of an array of new products the pharma hopes will offset patent expirations for current top sellers.

Pharmaceutical Technology
JUNE 9, 2023
The US Food and Drug Administration (FDA) has granted approval to commence commercial production at Bristol Myers Squibb’s new advanced cell therapy manufacturing facility in Devens, Massachusetts. Today’s approval underscores our commitment to deliver our transformational CAR T cell therapies to more patients.”
Pharmaceutical Technology
JUNE 12, 2023
It noted that the injection’s latest approval will help reduce the recent supply issues for the product in the country. This latest move marks Milla Pharmaceuticals’ second ‘first cycle’ FDA approval for an abbreviated new drug application (ANDA) and second paragraph IV filing.

Bio Pharma Dive
SEPTEMBER 17, 2022
The therapy, called Skysona and cleared to treat cerebral adrenoleukodystrophy, is the product of more than a decade of work by Bluebird. It will cost $3 million.

XTalks
NOVEMBER 28, 2023
This liquid formulation of metronidazole is the sole FDA-approved liquid option, offering a groundbreaking prescribing alternative for patients encountering difficulties in swallowing or facing taste-related obstacles. With a 24-month shelf life and no need for refrigeration, Likmez provides a convenient option for patients.

Pharmaceutical Technology
MARCH 27, 2023
It is claimed to be the first and only therapy to receive approval in the US for the treatment of APDS, a rare and progressive primary immunodeficiency. After assessing the New Drug Application (NDA) under priority review, the FDA granted the approval based on the data obtained from a Phase II/III clinical trial.
Pharmaceutical Technology
MAY 25, 2023
Yuflyma represents the company’s fifth biosimilar and second anti-TNF biosimilar to receive US FDA approval. The regulatory approval was based on a comprehensive data package of preclinical, analytical and clinical trials. It will be offered to patients in prefilled syringe and autoinjector administration options.

XTalks
JANUARY 2, 2024
Filsuvez topical gel is a sterile botanical drug product designed for topical use, containing birch triterpenes within an oil base. For instance, Vyjuvek , the first FDA-approved gene therapy for DEB, is priced at $24,250 per vial. Regarding the price of Filsuvez, it has not yet been publicly disclosed by the company.
XTalks
NOVEMBER 30, 2023
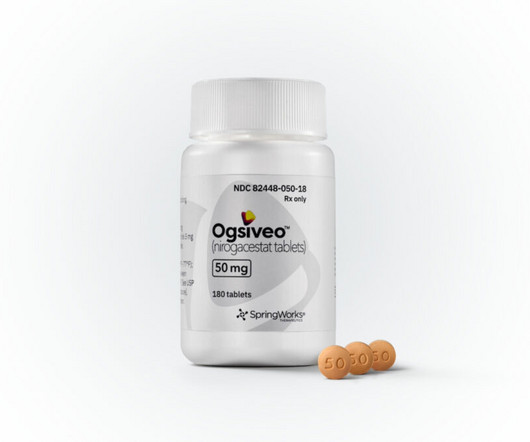
Pfizer spinout SpringWorks Therapeutics’ Ogsiveo (nirogacestat) has received US Food and Drug Administration (FDA) approval for the treatment of desmoid tumors, an ultra-rare subtype of non-cancerous soft tissue sarcomas that can cause severe pain and disfigurement.

Pharmaceutical Technology
JUNE 21, 2023
AGEPHA Pharma managing director Antonia Riel-Köllmann stated: “As the third generation of my family dedicated to developing high-quality European pharmaceuticals, it’s a privilege to bring this life-sustaining therapy, which represents the company’s first product launch in the US, to the global market. “We

Bio Pharma Dive
JULY 29, 2021
An injectable insulin from Viatris has become the first-ever biosimilar product that can be directly substituted for a marketed biologic, a long-awaited decision that could put pricing pressure on other diabetes drugs.

XTalks
DECEMBER 12, 2023
After receiving US Food and Drug Administration (FDA) approval for Fabhalta (iptacopan) last week for the treatment of the rare blood disorder paroxysmal nocturnal hemoglobinuria (PNH), Novartis presented trial data yesterday showing the drug’s promise in another indication.
Pharma Mirror
NOVEMBER 24, 2020
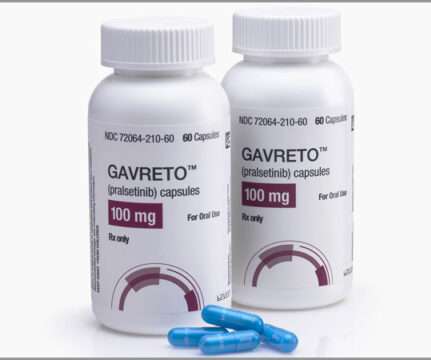
Catalent, the leading global provider of advanced delivery technologies, development, and manufacturing solutions for drugs, biologics, cell and gene therapies, and consumer health products, today announced that it had entered into a commercial supply agreement with Blueprint Medicines following FDA approval of GAVRETO™ (pralsetinib).
Pharmaceutical Technology
MARCH 1, 2023
In the last few years, there have been growing calls to onshore the manufacturing of certain pharma products, including active pharmaceutical ingredients (API), to ensure a smooth supply and to minimise shortages in the European Union (EU). You can also subscribe here to receive email notifications when a new issue is available.
XTalks
APRIL 24, 2024
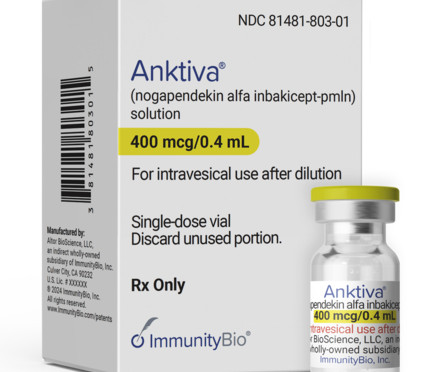
ImmunityBio’s Anktiva (N-803, or nogapendekin alfa inbakicept-pmln) along with the Bacillus Calmette-Guérin (BCG) vaccine has won US Food and Drug Administration (FDA) approval for the treatment of non-muscle invasive bladder cancer (NMIBC). Anktiva, a first-in-class IL-15 agonist immunotherapy, is ImmunityBio’s first approved product.

BioTech 365
DECEMBER 19, 2021
FDA Approves Telix’s Prostate Cancer Imaging Product, Illuccix® FDA Approves Telix’s Prostate Cancer Imaging Product, Illuccix® MELBOURNE, Australia and INDIANAPOLIS, Dec.
Pharmaceutical Technology
JUNE 13, 2023
The US Food and Drug Administration (FDA) has approved Neobiosis’ investigational new drug (IND) application for ViXome to treat post-Covid-19 syndrome (also known as long Covid). ViXome is an acellular product derived from amniotic fluid.

JAMA Internal Medicine
JULY 27, 2023
This Special Comunication examines relevant legal precedents on the Centers for Medicare & Medicaid Services’ approaches to limit coverage and recent decisions Medicare has issued affecting coverage for US Food and Drug Administration-regulated products.

Pharmaceutical Technology
DECEMBER 14, 2022
Harrow has signed a binding agreement to acquire exclusive US commercial rights to five ophthalmic products of Novartis. These products, namely, Ilevro, Vigamox, Maxidex, Nevanac and Triesence, have received approval from the Food and Drug Administration (FDA).

XTalks
OCTOBER 22, 2021
Boehringer Ingelheim’s Cyltezo has become the second interchangeable biosimilar approved by the FDA, and the first approved interchangeable monoclonal antibody. In July 2021, the agency approved the first interchangeable biosimilar product, Semglee (insulin glargine-yfgn) for the treatment of diabetes.

XTalks
FEBRUARY 15, 2023
Polarean is a medical imaging company focused on improving the state of lung imaging that recently received FDA approval for its drug-device combination product, Xenoview. Tune into the episode to learn more about the FDA approval of Xenoview, including the journey to its approval.

Pharmaceutical Technology
FEBRUARY 21, 2024
WuXi ATU received FDA approval to commence the analytical examination and production of Iovance’s Amtagvi at its Philadelphia site.
XTalks
JUNE 26, 2023
Surmodics will manufacture and supply the device and realize revenue from product sales to Abbott as well as a share of profits from Abbott’s third-party sales. It represents a major milestone in our efforts to develop next-generation products to help millions of people affected by peripheral artery disease and the physicians that treat them.”

XTalks
JULY 25, 2023
Cyfendus functions by stimulating the production of antibodies targeted against the protective antigen protein. Efficacy of Cyfendus The FDA approval of the Cyfendus anthrax vaccine is grounded in a series of studies supported by the US government and carried out by Emergent.
Pharmaceutical Technology
JUNE 15, 2023
By eliminating formulation steps common with other pemetrexed products, we are improving provider efficiencies while reducing the risk of medication errors. The product will be offered in 500mg/50mL,100mg/10mL and 1,000mg/100mL vial sizes.
Drug Discovery World
APRIL 4, 2024
The US Food and Drug Administration (FDA) has approved Vafseo (vadadustat) Tablets for the treatment of anaemia due to chronic kidney disease (CKD) in adults who have been receiving dialysis for at least three months. Vafseo is now approved in 37 countries.

XTalks
JANUARY 4, 2023
MediWound, a biopharmaceutical company focused on biotherapeutic solutions for tissue repair and regeneration, announced that the US Food and Drug Administration (FDA) approved their orphan biological product NexoBrid (anacaulase-bcdb) for the removal of eschar in adults with deep partial-thickness and/or full-thickness thermal burns.
Drug Discovery World
MARCH 22, 2024
The effects of ET-1 bear many similarities with the pathophysiology of hypertension, and ET-1 is a major driver of aldosterone production. ” The post FDA approval represents ‘transformational progress’ in hypertension appeared first on Drug Discovery World (DDW).
XTalks
SEPTEMBER 14, 2022
Daxxify was FDA-approved for similar cosmetic purposes as Botox and other neuromodulators like Dysport and Xeomin. Used for both cosmetic and therapeutic cases, Botox is a US Food and Drug Administration (FDA)-approved injection of botulinum toxin, a neurotoxic protein that can effectively paralyze the facial muscles.

BioTech 365
MARCH 29, 2021
FDA Approvals Strengthen Octapharma USA Pediatric Critical Care Product Portfolio FDA Approvals Strengthen Octapharma USA Pediatric Critical Care Product Portfolio Octaplas™ and fibryga® receive new product labeling following FDA’s approval of BLA supplements to update therapy research FDA expands fibryga® … Continue reading →

Pharmaceutical Technology
AUGUST 5, 2022
Thus, the biosimilar presented a similar efficacy to the reference product. All in all, following rigorous testing procedures, results for the safety and efficacy of Cimerli were highly promising, meeting the FDA’s meticulous standards, which ultimately led to its approval and prospective launch in October this year.
Drug Discovery World
MARCH 26, 2024
1 were consistent with the titer levels associated with efficacy in prior clinical trials of adintrevimab (ADG20), the parent mAb for VYD222, and other monoclonal antibody products. 1 is currently the dominant variant circulating in the US according to estimates from the Centers for Disease Control and Prevention (CDC).

Pharmaceutical Technology
JUNE 1, 2023
Dipharma is a pioneer in developing improved generic pharmaceutical products for rare diseases: our desire to innovate and our engagement do not stop, but every day we continue to seek new and better solutions for patients around the world.”
XTalks
SEPTEMBER 6, 2022
On Friday, Azurity Pharmaceuticals announced that the US Food and Drug Administration (FDA) approved their innovative oral liquid formulation Konvomep (omeprazole and sodium bicarbonate for oral suspension). Patients are our priority, and our purpose is to bring them new formulations that help them benefit from established medicines.

pharmaphorum
JANUARY 26, 2022
Immunocore has secured a piece of biotech industry history, becoming the first company to get an FDA approval for a cancer therapeutic based on T cell receptor (TCR) technology. It will be the UK biotech’s first commercial product, coming more than 14 years after it spun out of the UK subsidiary of Germany’s Medigene.

Pharmaceutical Technology
OCTOBER 31, 2023
The acquisition of the oral methotrexate solution, marketed as Jylamvo, marks the second FDA-approved oncology product in Shorla’s portfolio.

XTalks
JANUARY 17, 2024
Brain technology company Darmiyan has received De Novo US Food and Drug Administration (FDA) approval for its first-in-class dementia test BrainSee. BrainSee is a software as a service (SaaS) platform powered by AI that can assist physicians in determining the prognosis of patients who have amnestic mild cognitive impairment (aMCI).

XTalks
NOVEMBER 10, 2023
The US Food and Drug Administration (FDA) has approved Takeda Pharmaceuticals’ Adzynma, the first recombinant protein product for prophylactic (preventive) or on‑demand enzyme replacement therapy (ERT) in adult and pediatric patients with congenital thrombotic thrombocytopenic purpura (cTTP), an ultra-rare blood clotting disorder.

Drug Discovery World
JUNE 23, 2023
“If EMBARK confirms the benefits seen in our prior trials, Sarepta will move rapidly to submit a BLA supplement to expand the approved label as broadly as good science permits.” The post FDA approves first gene therapy for Duchenne muscular dystrophy appeared first on Drug Discovery World (DDW).

XTalks
DECEMBER 13, 2023
While Lyfgenia delivers a functional copy of hemoglobin A to replace the abnormal one in hematopoietic (blood) stem cells via a lentiviral vector, Casgevy edits the HBB gene using CRISPR technology to increase the production of fetal hemoglobin in blood stem cells.

Medical Xpress
NOVEMBER 17, 2022
Americans could soon be eating chicken that's grown in a lab from cultured animal cells, rather than raised at a farm or facility.

Camargo
JANUARY 31, 2020
By identifying the proper product category, we worked with an international company to correctly reposition its drug-device combination product for FDA approval and drive market penetration. The post Combination Product Expertise and Target Market Knowledge Create Commercial Success appeared first on Camargo.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content