Novartis buys a preclinical biotech and its RNA drug technology
Bio Pharma Dive
JULY 17, 2023
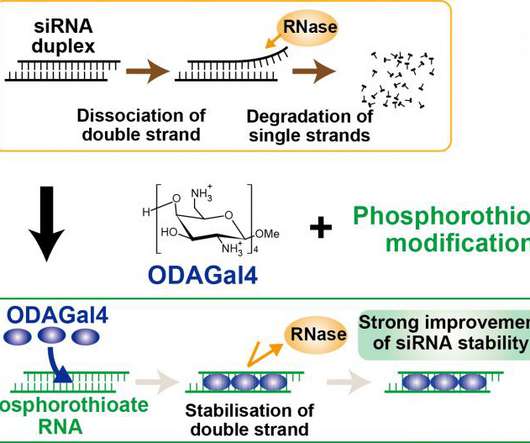
For $500 million, Novartis will acquire DTx Pharma and its preclinical neurological disease drugs, marking the Swiss company’s latest investment in gene-silencing medicines.





























Let's personalize your content