This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers advance knowledge on debilitating genetic disease
A team of researchers at the University of Georgia has identified the source of several symptoms in patients suffering from familial dysautonomia (FD), a rare and debilitating genetic disease that primarily affects children. This new knowledge could lead to improved treatments to mitigate symptoms and improve the overall quality of life for patients.
FD impairs the functioning of several nervous system branches simultaneously, limiting the ability of nerve cells throughout the body to work properly. Symptoms vary but typically emerge in early infancy as poor growth and feeding issues, then progress throughout a patient's lifetime to include developmental delays, frequent pneumonia and poor control of blood pressure, body temperature and breathing. As a result, FD patients typically have shorter life expectancies.
Researchers hope that by identifying underlying causes of the disease, they can better inform medical interventions for FD, as well as similar conditions like cardiovascular disease, heart failure and stress-related disorders like anxiety.
"In conducting this research, we hope to not only unlock answers about FD but trigger research into a whole haystack of diseases—specifically, what causes them," said Nadja Zeltner, an assistant professor in UGA's Center for Molecular Medicine and the project's principal investigator.
These findings were published in a recent issue of Nature Communications, in which Zeltner's Ph.D. advisee Hsueh-Fu Wu served as first author.
One particularly concerning aspect of FD is the "dysautonomic crisis"—extreme episodes that frequently result in vomiting, severe shifts in heart rate and blood pressure, and personality changes. These events, long understudied, form the core of Zeltner's research.
"The dysautonomic crisis is triggered by stress," Zeltner explained. "Positive stress, negative stress— it doesn't matter. Just imagine a panic attack that doesn't really go away."
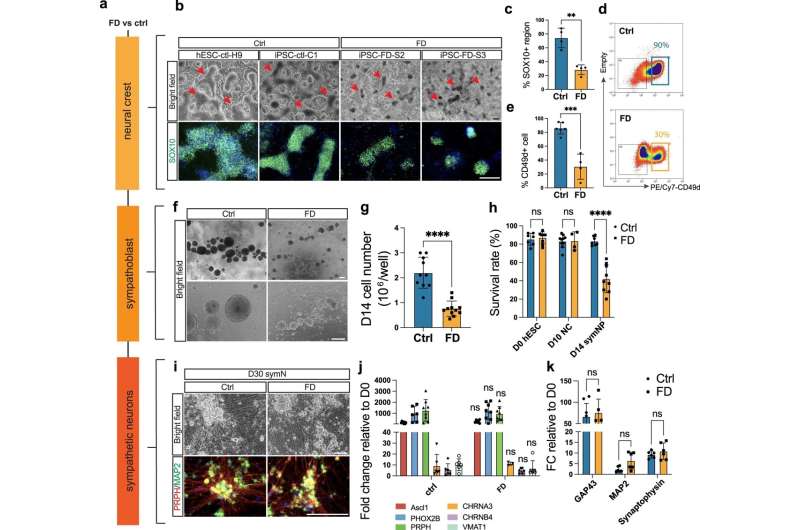
The symptoms associated with these episodes are controlled by sympathetic neurons, the same nerves responsible for triggering fight-or-flight reactions. In her research, Zeltner used stem cells from FD patients that were converted into sympathetic nerve cells to better understand why these cells do not work and why they produce such severe symptoms in patients. This work yielded significant results that previously had not been demonstrated by research.
"Researchers originally thought that these neurons die and leave no trace behind," Zeltner said. "But we found that they're actually hyperactive. Nobody had shown that before."
Hyperactivity is a stark departure from previous findings. It was determined that sympathetic neurons in FD patients cause such unpleasant symptoms because they activate tissues within the body to an excessive degree. This not only helps to explain why patients react the way they do to stressful events, but also helps unlock questions about potential treatments.
In her study, Zeltner tested a series of drugs to see if they helped reduce hyperactivity associated with FD. This began with testing dexmedetomidine, which currently used for patients in dysautonomic crisis; the drug was determined to be effective precisely because it reduced the hyperactivity.
"That was really cool, to see how this drug is tied to effectiveness," Zeltner said. "Because of this research, we can now help by explaining more about the mechanism behind this drug."
Zeltner then studied a number of other drugs known to reduce hyperactivity, several of which also proved successful in mitigating hyperactivity associated with FD. This potentially exciting development might shed new light on how to control symptoms in patients, but Zeltner warned that it's too early to know how this research might impact treatment.
"We're pretty far from being able to give new drugs to patients, for example," Zeltner said. "But it's a good start. What this research has done is provide a platform for previously unconsidered drugs to be tested, and that's a really valuable tool."
This research also has the potential to unlock answers about a wide range of stress-induced disorders, ranging from hypertension to heart failure. That's because understanding the stress mechanisms in FD patients, especially in relation to the sympathetic nervous system, provides a strong model for understanding the effects of stress upon people in a variety of conditions.
"Stress is a very omnipresent problem in our daily lives," Wu said. "Hopefully we can apply this knowledge from this model system to other stress-related metabolic diseases. That's the goal."
More information: Hsueh-Fu Wu et al, Norepinephrine transporter defects lead to sympathetic hyperactivity in Familial Dysautonomia models, Nature Communications (2022). DOI: 10.1038/s41467-022-34811-7