This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Novel test could ensure newborns with a serious genetic disease receive essential treatment
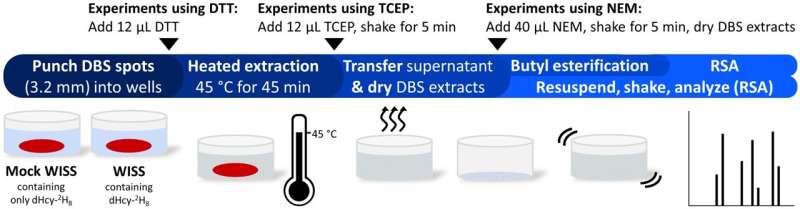
Scientists have developed a test that could greatly improve quality of life for infants with homocystinuria (HCU), a congenital disease that—if not treated early—causes serious complications. Research demonstrating the efficacy of this test was published today in Clinical Chemistry.
HCU impedes an infant's ability to metabolize the amino acid methionine, which is a component of many proteins, such as those found in breastmilk. This leads to a pathological increase in levels of methionine and another amino acid known as homocysteine, causing severe complications if left untreated. These complications range from eye and skeletal issues to vascular abnormalities and intellectual disabilities.
The good news is that early detection and treatment of HCU can prevent these complications. In light of this, since 2006, the U.S. Department of Health and Human Services has included HCU on the list of disorders for which newborns should be screened. However, current tests only measure levels of methionine, which are often still low when newborn screening occurs. As a result, it's estimated that these tests miss around 50% of HCU cases, which are then at high risk of going untreated.
In an effort to remedy this, a group of researchers led by Konstantinos Petritis, Ph.D., at the Centers for Disease Control and Prevention, has developed and validated a newborn screening test for HCU that works by measuring homocysteine levels. In infants with HCU, homocysteine levels usually rise before methionine levels, and they almost always rise during the first few days of life when newborn screening is performed, making homocysteine a better early marker of this disease.
To evaluate the test's performance, Petritis's team used it to screen residual newborn screening specimens from infants who had already received diagnoses. One hundred of these samples were from healthy patients; 50 came from HCU-negative infants receiving total parenteral nutrition (TPN), which is given to premature babies in the NICU; and 2 samples were from HCU-positive patients.
The test successfully distinguished between the healthy and HCU-positive samples. It also accurately classed the TPN samples as HCU-negative, which is noteworthy because another problem with methionine tests for HCU is that they produce false positives in babies receiving TPN.
"Here we present the only flow injection analysis-tandem mass spectrometry first-tier newborn screening method that directly quantifies total homocysteine from dried blood spots," said Petritis.
"The ability to screen total homocysteine during first-tier newborn screening is a significant step toward reducing HCU false-negative rates, which will enable early identification and intervention to reduce HCU-associated morbidity and mortality."
More information: C Austin Pickens et al, Multiplexing Homocysteine into First-Tier Newborn Screening Mass Spectrometry Assays Using Selective Thiol Derivatization, Clinical Chemistry (2023). DOI: 10.1093/clinchem/hvad007

















