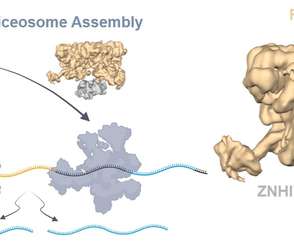
Rare genetic disease caused by mutations in protein that controls RNA metabolism
Scienmag
MAY 7, 2021
Credit: UPMC PITTSBURGH, May 7, 2021 – In a paper published today in Nature Communications, an international group of collaborators led by researchers at UPMC Children’s Hospital of Pittsburgh have identified a genetic cause of a rare neurological disorder marked by developmental delay and loss of coordination, or ataxia.








































Let's personalize your content