Tome Biosciences debuts with $213M and a new way to edit the genome
Bio Pharma Dive
DECEMBER 12, 2023
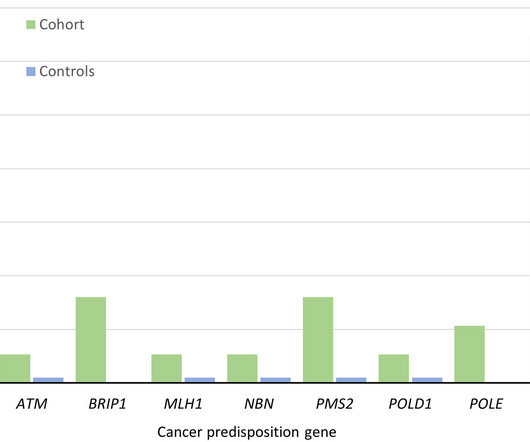
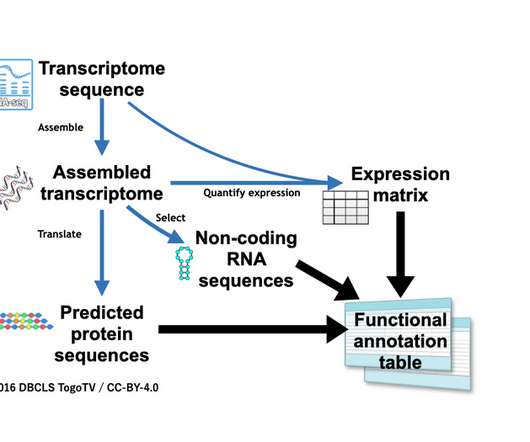
Based on the work of MIT scientists, the well-funded startup is developing ways to insert large sizes of genetic material anywhere in the genome without damaging or breaking DNA.






































Let's personalize your content