This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Innovative in vitro eye irritation test to replace standard animal testing

In the worst-case scenario, getting chemicals in your eyes can lead to blindness. Until now, the eye irritancy potential of chemical substances has had to be assessed using the Draize test on live rabbits—a standard implemented worldwide.
Researchers at the Translational Center for Regenerative Therapies TLC-RT of the Fraunhofer Institute for Silicate Research ISC want to work with partners to replace animal testing: Tissue models of the human cornea cultivated in the laboratory are expected to completely replace the Draize test. It is hoped that this new test procedure will become the future standard.
The human eye is a sensitive organ—its anatomy and position mean that it is particularly exposed to external risks. It can be damaged through contact with chemical substances, such as those present in many household products. A drop of acid is enough to cause harm to the cornea and leave scarring. A few drops of alkaline liquids can even cause the cornea to be cloudy forever.
For this reason, experts have been testing chemicals for their potential to cause eye irritation since 1944 using the Draize test. This involves dropping such substances into the eyes of rabbits. The chemical substances are then classified after a few days as follows: as GHS (Globally Harmonized System of Classification and Labeling of Chemicals) Category 1 for irreversible damage, GHS Category 2 for reversible damage or, for non-harmful chemicals, as chemicals that do not require labeling.
Scientists around the world are working hard on an alternative to harmful toxicological testing on animals. Until now, however, there has been no reliable way to differentiate between reversible and irreversible damage, meaning that completely replacing the Draize test was not possible. As part of the ImAi project (see box), researchers at the Fraunhofer Translational Center for Regenerative Therapies TLC-RT of Fraunhofer ISC in Würzburg and partners from industry and research are currently developing an impedance-based in vitro testing system that enables this differentiation. Once it is validated, it will be submitted to the Organization for Economic Co-operation and Development (OECD) to be established as a new test guideline.
Impedance-based eye damage test
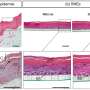
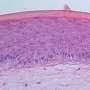
The new testing procedure combines a modified model of a cornea based on human tissue models with non-invasive impedance spectroscopy. "At the laboratory, we cultivate human tissue modeled on the anterior part of the cornea. The tissue cells are encased by a cell membrane, the chemical composition of which allows it to act as an electric insulator. The cells form one or more closed layers, creating measurable resistance. The barrier properties of the epithelia—the outermost layer of the cornea—can be determined by measuring the electric resistance," explains Dr. Christian Lotz, a scientist at the TLC-RT.
Now, if a test substance causes any damage to the eye, the barrier is destroyed, cells die, holes appear, and the resistance decreases. The electricity is able to flow freely once more. The more cell layers that die, the higher the decrease in resistance. In this way, the state of the tissue can be measured using the impedance spectrometer in an indirect—and therefore non-invasive—way, without destroying the tissue model. If the cell tissue is not damaged, resistance will be high. However, if the cells are destroyed, the electrical barrier will be broken.
Differentiating between reversible and irreversible damage is possible
"Contrary to other methods, our impedance-based eye damage test is non-destructive—we can continue using the same model, measuring and analyzing it to determine whether the tissue recovers over the course of seven or more days," says the expert in biomedicine. This is not the case for Category 1 toxic substances, but for Category 2 chemicals, the cell structure regenerates within seven days. This means that a clear classification can be made as to whether any damage is reversible or irreversible.
"Different chemicals can be applied to the in vitro tissue model over the course of a test, and we can even measure tissue regeneration. This is a novelty and something that has never been done before. It was previously not possible to ascertain whether the cell structure had recovered after seven days. Thanks to impedance spectroscopy, this can be easily achieved with our test."
The mobile impedance spectrometer for analyzing the in vitro tissue model is a quarter of the size of a thick book. As well as an electrode plate for measuring the electrical resistance, it comprises a cell culture plate with tissue wells for 24 cornea models as well as the electronic system. The device can be plugged into a laptop for the analysis. Given its small size, it is easy to transport it to the cell bank.
Multi-laboratory study forms the basis for new test guideline within the framework of the OECD
A training set of 70 substances—including acids, bases and other chemicals—from the various GHS categories were initially used for the test. The reproducibility of the test is currently being proven as part of a multi-laboratory study involving the German Federal Institute for Risk Assessment (BfR) and Goethe University Frankfurt. For this purpose, the laboratories are using an optimized, blinded validation set comprised of 30 test substances.
"With the help of the multi-laboratory study, we want to prove that the innovative, non-invasive measurement methodology can not only be used by Fraunhofer ISC but also by other research institutions," explains Lotz. The results will be used to decide whether a new, globally recognized test guideline that corresponds to the regulatory requirements will be developed within the OECD framework, meaning that it will be possible to make predictions on the effect of chemicals on human health and the environment without using animal testing. "We are confident that in around two to three years, a new, internationally recognized test guideline will be available as an alternative to animal testing."