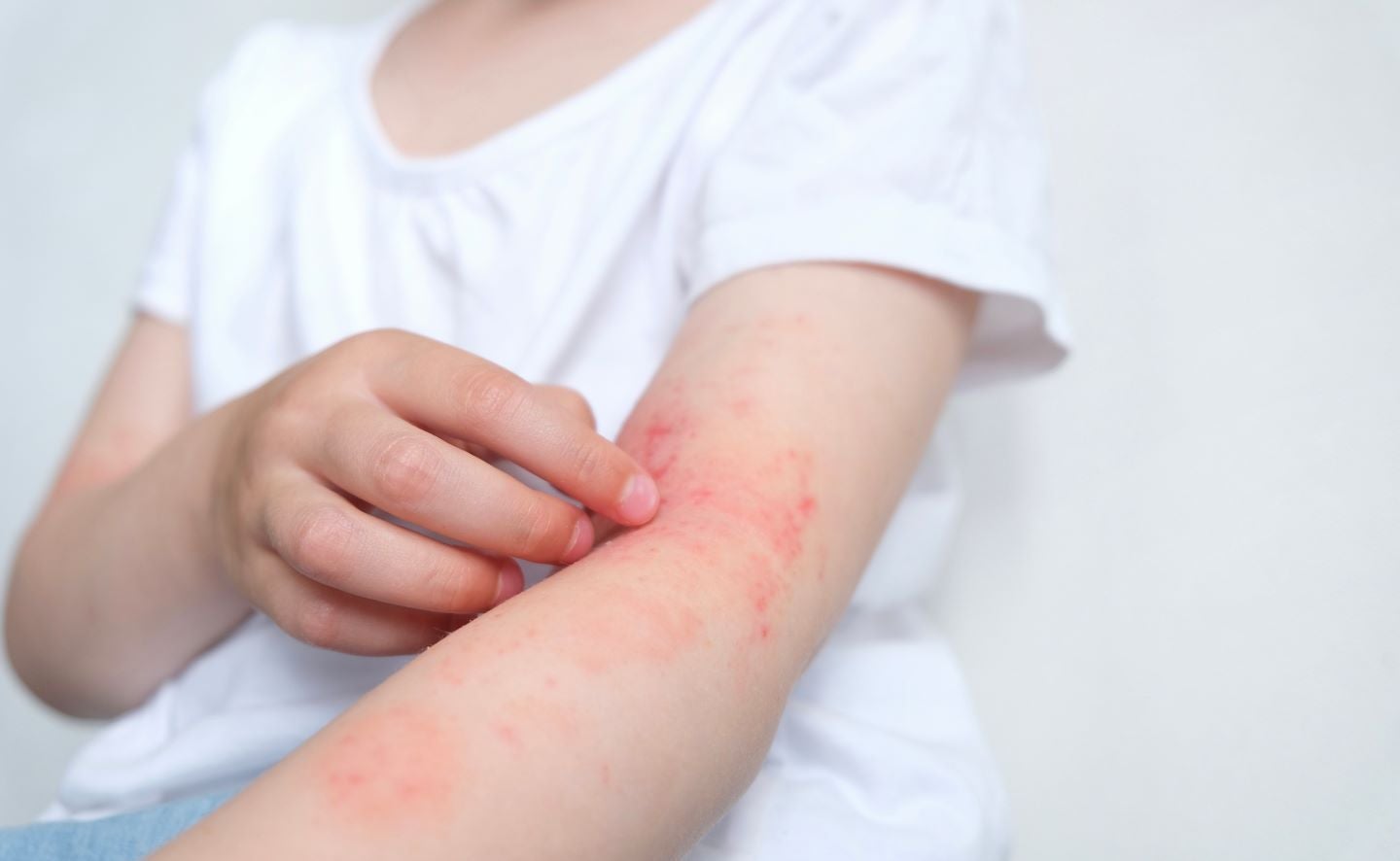
Astria Therapeutics has signed an exclusive global agreement to license Ichnos Sciences’ OX40 portfolio to treat atopic dermatitis (AD).
Astria will make a one-time upfront licence fee payment of $15m to Ichnos.
Ichnos is also entitled to receive $305m in milestone payments from Astria.
The milestone payments comprise $20m for clinical development and $285m for obtaining regulatory approval and commercial sales of licensed products, both for up to three indications.
Astria will make tiered royalty payments based on yearly net product sales to Ichnos.
See Also:
Ichnos is developing its OX40 portfolio for the potential treatment of AD and other allergic and immunological ailments.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataIt comprises the monoclonal antibody OX40 antagonists STAR-0310 and telazorlimab.
Astria has obtained an exclusive licence worldwide for these assets in all fields.
Astria will develop STAR-0310, a preclinical-stage programme for treating AD, and will explore further indications in the future.
The company also intends to file an investigational new drug application for the programme by the end of 2024.
Having gained regulatory approval, Astria will commence a Phase Ia clinical trial of STAR-0310 in healthy subjects in 2025.
Astria Therapeutics CEO Jill Milne stated: “We are very proud to add such a strong programme to our company that supports our vision of strategic growth for the future.
“We are building a pipeline of potential first-choice products that can improve the health and outcomes for allergy and immunology patients.”
In July 2023, the US Food and Drug Administration granted orphan drug designation for Ichnos’ ISB 2001 for multiple myeloma.





