Cristália signs licensing deal for Formosa’s APP13007
Pharmaceutical Technology
JANUARY 29, 2024
Cristália has signed an agreement to license exclusive rights to market Formosa Pharmaceuticals’ APP13007 in Brazil.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Pharmaceutical Technology
JANUARY 29, 2024
Cristália has signed an agreement to license exclusive rights to market Formosa Pharmaceuticals’ APP13007 in Brazil.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
DECEMBER 8, 2022
According to the revised deal, Daiichi Sankyo and Kite will transfer the Marketing Authorization for Yescarta to Gilead’s Japan subsidiary, Gilead Sciences K.K., The therapy’s sales and promotion works in Japan following the transfer of marketing authorisation will be carried out by the Kite Cell Therapy Business Unit at Gilead Sciences K.K.

Pharmaceutical Technology
JANUARY 2, 2023
South Korean biotechnology company Alteogen has signed an exclusive license agreement with Swiss company Sandoz to develop and market biosimilar products that are enabled by the former’s Hybrozyme technology. Additionally, the company will have an option to license Alteogen’s Hybrozyme technology for two more products.

Pharmaceutical Technology
DECEMBER 5, 2022
Women’s healthcare company TherapeuticsMD has sign ed agreements for licensing its products to the Mayne Pharma affiliate in the US. Additionally, TherapeuticsMD will grant Mayne the exclusive license to market Annovera in the US. TherapeuticsMD will also get a 20-year royalty stream linked to Mayne’s net product sales.

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 20, 2023
Empowering of the Central Drugs Standard Control Organisation (CDSCO) through centralisation of drug licensing may not be the path to improve the quality of drugs manufactured in the country both for domestic and international markets, says SME Pharma Industries Confederation (SPIC), the apex organisation of small and medium pharma industries in the (..)
Pharmaceutical Technology
JUNE 19, 2023
Bio-Thera Solutions and Biomm have entered a licensing and supply agreement for Bio-Thera’s BAT2206, a ustekinumab biosimilar. Biomm will gain exclusive rights for the distribution and marketing in Brazil of the proposed biosimilar to Janssen’s Stelara.

Pharmaceutical Technology
MARCH 25, 2024
An unnamed Chinese partner takes on AR1001 in an APAC Alzheimer’s market predicted to be worth $2.6bn by 2030
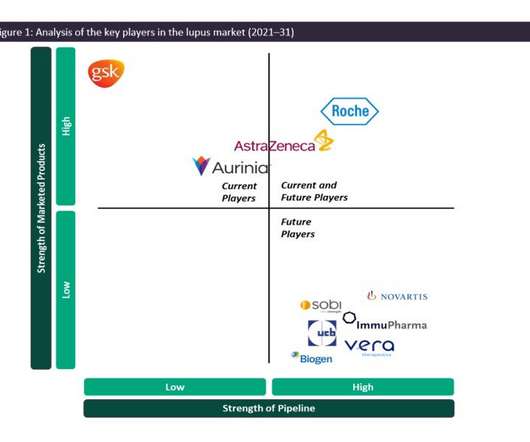
Pharmaceutical Technology
JANUARY 23, 2023
As such, the SLE and LN marketplace is dominated by generics, and GSK’s Benlysta and AstraZeneca’s Saphnelo are the only drugs that have gained marketing approval specifically for SLE in more than 50 years. In previous years, Benlysta has managed to grow the lupus market in terms of value, having generated approximately $492.9m

Pharmaceutical Technology
JUNE 27, 2023
The FDA has accepted a biologics license application (BLA) for Pfizer's fidanacogene elaparvovec to treat adults with haemophilia B.
Drug Discovery World
AUGUST 2, 2023
Renaissance Pharma has announced its first development programme focused on Hu14.18, a humanised anti-GD2 monoclonal antibody (mAb), licensed from St Jude Children’s Research Hospital for the treatment of newly diagnosed high-risk neuroblastoma. ” The post Renaissance Pharma licenses Hu14.18
Pharmaceutical Technology
JANUARY 23, 2023
Takeda has signed an exclusive licence agreement with HUTCHMED (China) and its subsidiary HUTCHMED to develop and market the latter’s fruquintinib. The China National Medical Products Administration (NMPA) approved fruquintinib for marketing in September 2018. The submission is planned to be completed in the first half of this year.
Pharmaceutical Technology
JULY 28, 2022
Melinta Therapeutics has signed a licence agreement with Cidara Therapeutics to facilitate a strategic collaboration for marketing the latter’s rezafungin in the US. The post Melinta and Cidara sign licensing deal for rezafungin appeared first on Pharmaceutical Technology.
Pharmaceutical Technology
AUGUST 18, 2023
Imugene's azercabtagene zarpreleucel (azer-cel) allo-CAR T cell therapy has shown a 58% ORR and 41% CR rate across all doses.
Bio Pharma Dive
AUGUST 1, 2023
Some on Wall Street believe the New York-based biotechnology company is now less likely to get acquired after agreeing to sell partial rights to its only marketed product.
BioPharma Reporter
APRIL 2, 2024
Ipsen is expanding its oncology pipeline by securing the global licensing rights to an antibody-drug conjugate (ADC) for solid tumors from Sutro Biopharma, its first drug in the category.

Pharmaceutical Technology
SEPTEMBER 27, 2023
The idiopathic pulmonary fibrosis (IPF) market is underserved with just two licensed pharmaceutical treatments approved by the FDA in 2014.

Pharmaceutical Technology
SEPTEMBER 11, 2023
The agreement allows Sandoz exclusive marketing rights for the inflammatory bowel disease therapy in Europe and North America.
Pharmaceutical Technology
OCTOBER 23, 2023
On 2 October, Eli Lilly announced that the US Food and Drug Administration (FDA) had issued a complete response letter (CRL) regarding the biologic license application (BLA) for its anti-interleukin (IL)-13 biologic lebrikizumab for the treatment of adults and adolescents (12 and older) with moderate-to-severe atopic dermatitis (AD).

Pharmaceutical Technology
APRIL 27, 2023
The European Commission’s (EC) long-anticipated pharma reform plans in the European Union have finally been unveiled , indicating a focus on improving access to medicines across the bloc while cutting down on market exclusivity.
Pharmaceutical Technology
MAY 24, 2023
Y-mAbs Therapeutics has received marketing authorisation for Danyelza (naxitamab-gqgk) 40mg/10mL injection from the Brazilian Health Regulatory Agency, Agência Nacional de Vigilância Sanitária, to treat high-risk neuroblastoma. The therapy is given three times a week and repeated every four weeks.

Pharmaceutical Technology
MAY 22, 2023
Biogen and Eisai have submitted a marketing authorisation application (MAA) to the UK’s medicines and healthcare products regulatory agency (MHRA) for lecanemab to treat early Alzheimer’s disease (AD). The regulator has also designated the therapy for the innovative licensing and access pathway (ILAP).
Pharmaceutical Technology
MAY 3, 2023
Aurinia Pharmaceuticals has received marketing authorisation from the Swiss Agency for Therapeutic Products (Swissmedic) for Lupkynis (voclosporin), along with a background immunosuppressive therapy. They also maintained a stable estimated glomerular filtration rate (eGFR) over three years.

BioPharma Reporter
OCTOBER 26, 2023
The biopharmaceutical industry witnessed a 400% growth in total deal value of antibody-drug conjugate (ADC) licensing agreements from 2017-2022 and reached a peak of $16.6 billion in 2022, reveals data and analytics company GlobalData.

pharmaphorum
JANUARY 9, 2023
Swiss biotech Stalicla has continued to diversify its pipeline from a focus on autism therapies with a deal to license Novartis’ mavoglurant, in clinical trials for cocaine use disorder (CUD) as well as neurodevelopmental disorders (NDD). The market potential of the CUD and NDD indications alone could top 2 billion euros ($2.12

Fossil Remedies
OCTOBER 16, 2022
In a pharma business, you have a distribution network like a pharma manufacturing company, pharma marketing company, C&F agents, wholesalers/distributors/stockiest, retailers, and pharmacies. To launch the best PCD pharma franchise company, you will have to acquire the necessary licenses from authorities. Wholesale Drug License.
Bio Pharma Dive
DECEMBER 14, 2021
Fitusiran, which Sanofi licensed from Alnylam, could finally get to market after trial delays and safety concerns slowed its progress. But gene therapies and other new medicines might provide competition.

Pharmaceutical Technology
MARCH 17, 2023
Pharmanovia and Aeterna Zentaris have entered an exclusive licensing agreement for the latter’s medicine Ghryvelin (macimorelin). Under the deal, Pharmanovia will acquire the exclusive rights and license to commercialise Ghryvelin in the EEA and the UK from Consilient Health.

BioPharma Reporter
OCTOBER 31, 2023
The French biotech company Vect-Horus has licensed its technology to the Danish big pharma Novo Nordisk to deliver drug cargoes to undisclosed disease targets in three programs.
Pharmaceutical Technology
JUNE 5, 2023
Pint-Pharma will obtain and maintain all marketing authorisations and will commercialise BESREMi in the region. In 2019, the European Medicines Agency approved the therapy, which has also been licensed in Japan, Taiwan and South Korea. It holds orphan drug designation in the US to treat PV.

BioPharma Reporter
SEPTEMBER 25, 2023
MC2 Therapeutics has acquired the exclusive licensing rights for Regranionâs RGRN-305, a new treatment for hidradenitis suppurativa (HS).

Pharmaceutical Technology
MAY 8, 2023
Under the terms of the license and commercialisation deal, Dr. Reddy’s will get licence for the development and commercialisation of toripalimab in India, Panama, Peru, Colombia, South Africa, Brazil, Argentina, Chile, Uruguay, and Mexico.

BioPharma Reporter
JANUARY 6, 2023
Synaffix, a Netherlands based company providing clinical-stage platform technology for the development of antibody-drug conjugates (ADCs), has signed off on two new licensing deals this week.

Drug Discovery World
JUNE 1, 2023
A Nature publication confirmed that there are currently 12 FDA-approved ADCs on the market, and nine of these secured FDA approval in the past six years 2. DDW’s Megan Thomas explores three trends in the market. Varying sources suggest that China has been one to watch over recent years and continues to be going forward.

pharmaphorum
NOVEMBER 15, 2022
Lantheus’ pipeline-building drive in radiopharma has continued with a $260 million upfront deal to license rights to two oncology candidates from POINT Biopharma. The post Lantheus on POINT in radio-oncology with $2bn licensing deal appeared first on. billion in milestone payments.

Pharmaceutical Technology
JANUARY 19, 2023
Acute myeloid leukemia (AML) is part of a market of blood malignancies that commercial cell therapies have not managed to penetrate yet. None of these products are in a registrational trial, and as such, GlobalData does not anticipate their entry into the AML market earlier than 2028.

pharmaphorum
NOVEMBER 23, 2021
Sanofi has bolstered its push into mRNA-based therapies with a new licensing deal – but not as might be expected with some up-and-coming biotech company. The post Chinese tech giant Baidu licenses mRNA algorithm to Sanofi appeared first on.

BioTech 365
JUNE 25, 2021
Aurinia Announces Licensing Partner Otsuka Filed Initial Marketing Authorization Application (MAA) for Voclosporin with the European Medicines Agency (EMA) Aurinia Announces Licensing Partner Otsuka Filed Initial Marketing Authorization Application (MAA) for Voclosporin with the European Medicines Agency (EMA) VICTORIA, British … Continue reading (..)

BioTech 365
JUNE 9, 2021
World Pancreatic Cancer Market Spotlight 2021-2031: Key Marketed and Pipeline Drugs, Events, Clinical Trials, Regulations, Disease Prevalence, Licensing and Acquisition, Revenue – ResearchAndMarkets.com World Pancreatic Cancer Market Spotlight 2021-2031: Key Marketed and Pipeline Drugs, Events, Clinical Trials, Regulations, Disease Prevalence, (..)
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?



Let's personalize your content