CMO Moves: Regulatory catalysts for drug manufacturing-December
Pharmaceutical Technology
DECEMBER 12, 2022
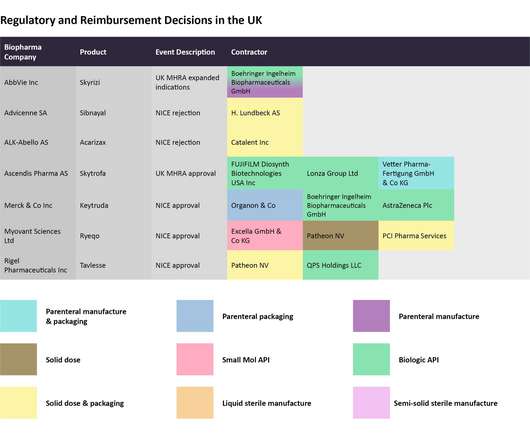
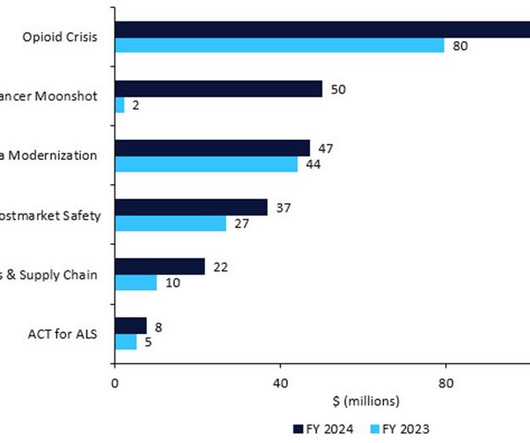
In this last 2022 edition of the series, which started in June , Pharmaceutical Technology is tracking major trial announcements and decisions by regulators and reimbursement agencies that have occurred since mid-October, as well as their potential impact on manufacturing plans. Iomab-B met the durable complete remission endpoint.
















































Let's personalize your content