AstraZeneca to supply the US with up to half a million additional doses of the potential COVID-19 antibody treatment AZD7442
The Pharma Data
MARCH 16, 2021
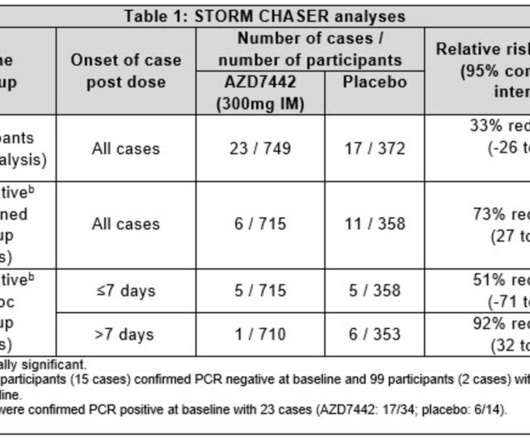
AstraZeneca has modified an existing agreement with the US Government to supply up to 500,000 additional doses of AZD7442, a long-acting antibody (LAAB) combination which is in late-stage development for the prevention and treatment of COVID-19. AZD7442 is currently being assessed in five late-stage prevention and treatment trials.
























Let's personalize your content