Intravacc gets NIAID contract for intranasal gonorrhoea vaccine development
Pharmaceutical Technology
OCTOBER 6, 2022
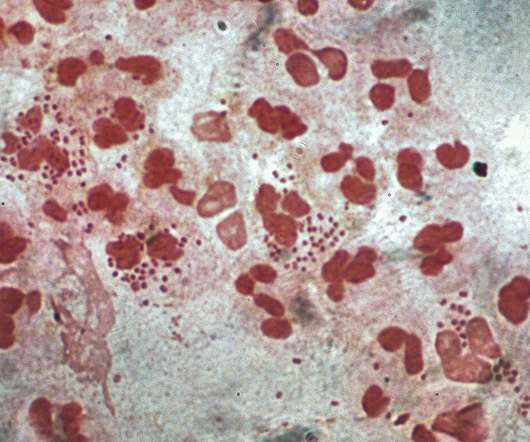
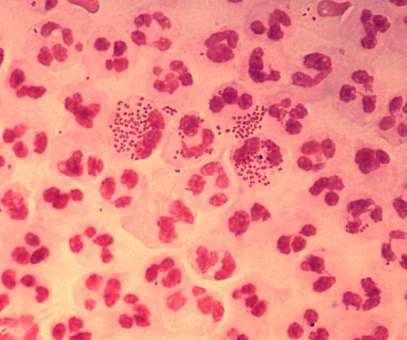
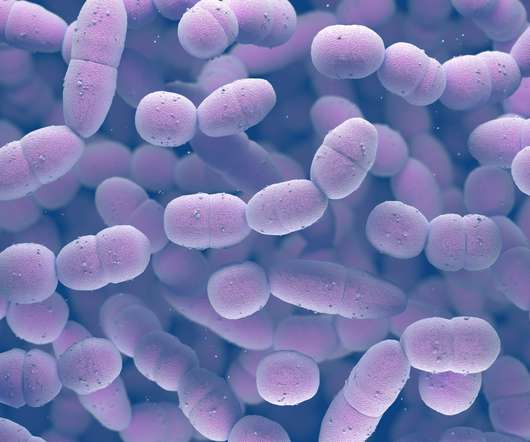
Intended for intranasal administration, NGoXIM was demonstrated to be effective in animal models and elicited a durable and cross-protective immune response in proof-of-concept (PoC) studies. . This trial will evaluate the safety of the vaccine and generate efficacy data.
















Let's personalize your content