Erasca receives fast track designation for glioblastoma therapy
Pharmaceutical Technology
MAY 2, 2023
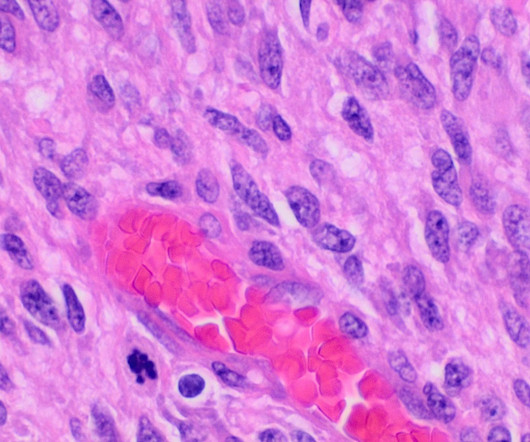
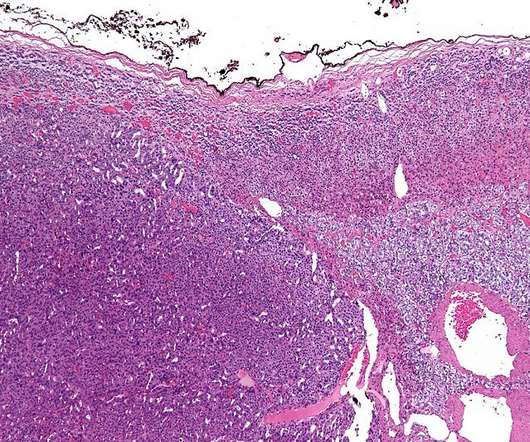
Erasca has received the US Food and Drug Administration’s (FDA) fast track designation (FTD) for ERAS-801 to treat adults with glioblastoma (GBM) with epidermal growth factor receptor (EGFR) gene alterations. A team of three cancer researchers, Michael Jung, Timothy Cloughesy and David Nathanson, designed and developed ERAS-801.











Let's personalize your content